Novel insights into the differential functions of Notch ligands in vascular formation
Journal of Angiogenesis Research. 2009;
Received: 19 August 2009 | Accepted: 16 November 2009 | Published: 16 November 2009
Vascular Cell ISSN: 2045-824X
Abstract
The Notch signaling pathway is a critical component of vascular formation and morphogenesis in both development and disease. Compelling evidence indicates that Notch signaling is required for the induction of arterial-cell fate during development and for the selection of endothelial tip and stalk cells during sprouting angiogenesis. In mammals, two of the four Notch receptors (Notch1 and Notch4) and three of the five Notch ligands (Jagged1, Dll1, and Dll4) are predominantly expressed in vascular endothelial cells and are important for many aspects of vascular biology. During arterial cell-fate selection and angiogenesis, the roles of Notch1 and Notch4 are thought to be similar, and the function of Dll4 is well-characterized. However, the molecular mechanisms that determine the functional similarities and differences of Notch ligands in vascular endothelial cells remain largely unknown; consequently, additional research is needed to elucidate the ligand-specific functions and mechanisms associated with Notch activation in the vascular endothelium. Results from recent studies indicate that Dll1 and Dll4 have distinct roles in the specification and maintenance of arterial cell identity, while Dll4 and Jagged1 have opposing functions in tip- and stalk-cell selection during sprouting angiogenesis. This review will focus on the newly discovered, distinct functions of several Notch ligands in the regulation of blood vessel formation and will provide perspectives for future research in the field.
Introduction
Notch signaling is evolutionarily conserved and critical for cell-fate determination, differentiation, and many other biological processes [1]. The mammalian Notch signaling pathway is composed of four Notch receptors (Notch1-4) and five ligands (Jagged1 and 2 and Delta-like [Dll] 1, 3, and 4). All of the ligands are transmembrane-type proteins and, consequently, Notch signaling is often mediated by cell-cell interactions. Transmission generally occurs between neighboring cells that express high levels of either the receptor or the ligand, although receptor-ligand coexpression occurs in some cells, such as vascular endothelial cells. Over the last decade, numerous studies have demonstrated that Notch signaling is critically involved in vascular development and disease [2–6]. For example, Notch signaling is required for arterial cell-fate determination during embryonic development, and the Notch pathway controls both developmental and pathological angiogenesis by modulating the selection of endothelial tip and stalk cells in newly sprouting blood vessels. Regulation of the Notch pathway in blood vessels has been well characterized; however, the specific roles of each Notch ligand during vascular formation and morphogenesis are unknown. Recent studies provide insight into the distinct functions of Notch ligands in blood vessels, and this review summarizes the current understanding of how several ligands differentially activate Notch signaling in the vasculature.
Basic mechanisms of the Notch signaling pathway
Notch signaling is initiated by interactions between a Notch ligand expressed on the surface of one cell (the signaling cell) and a Notch receptor expressed on the surface of a neighboring cell (the receiving cell). Upon ligand binding, Notch is sequentially cleaved, and the Notch intracellular domain (NICD) is released into the cytoplasm. The NICD enters the nucleus, where it interacts with the transcription factor CSL (named after mammalian CBF1,
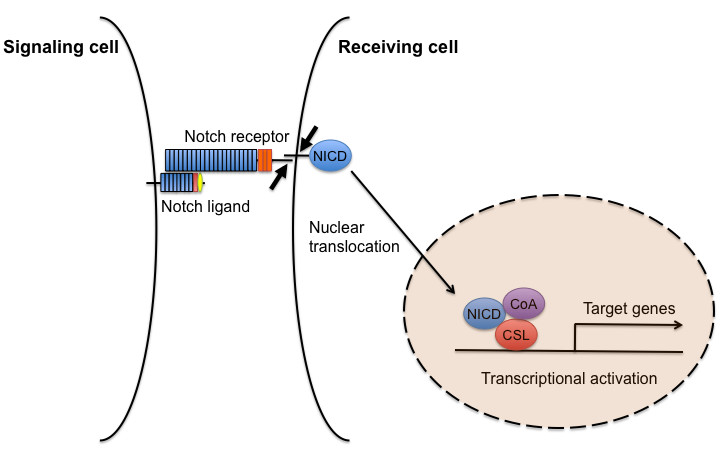
Figure 1
Figure 1 caption
A diagram of the canonical Notch signaling pathway. This schematic shows a simplified overview of the main components of Notch signaling. Upon Notch ligand binding, a two-step proteolysis cleavage process (small arrows) within the juxtamembrane region and transmembrane domain of the Notch receptor is catalyzed by a member of the disintegrin and metalloproteases (ADAMS) family and the γ-secretase containing complex, respectively, then the Notch intracellular domain (NICD) is released from the membrane and translocates to the nucleus, where it forms a transcriptional activation complex with CSL and coactivators (CoA), thereby inducing the transcription of target genes.
The extracellular domains of mammalian Notch ligands have several distinct features that participate in receptor binding (Figure 2). Their N-terminal regions contain a conserved module, and a second conserved module, the DSL (Delta/Serrate/LAG-2) domain, is located adjacent to the N-terminal region. Both Notch ligands and receptors contain multiple EGF-like repeats, and the ligands Jagged1, Jagged2, and Dll1 have tandem EGF repeats that form the DOS (Delta and OSM-11-like proteins) domain [8]. Jagged1 and Jagged2 also contain a cysteine-rich domain located between the EGF-like repeats and the transmembrane domain. Both the DSL and DOS domains are critical for receptor binding [9], and the structural diversity of Notch ligands is determined by the presence or absence of the cysteine-rich DOS domains.
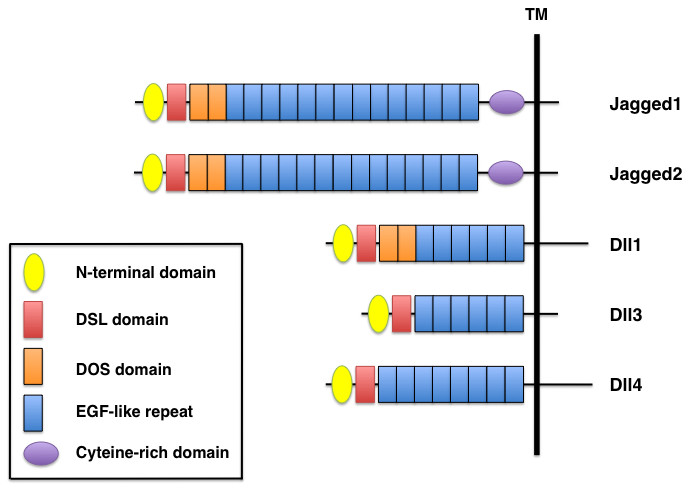
Figure 2
Figure 2 caption
Domain organization of mammalian Notch ligands. Five mammalian ligands are classified into two categories, Delta-like (Dll1, Dll3, Dll4) and Serrate-like (Jagged1, Jagged2), based on structural homology to the two Drosophila ligands, Delta and Serrate. All Notch ligands have an N-terminal domain, a DSL (Delta/Serrate/LAG-2) domain and EGF-like repeats. Jagged1 and Jagged2 contain a cysteine-rich domain, whereas Jagged1, Jagged2, and Dll1 have two DOS (Delta and OSM-11-like proteins) domains located immediately following the DSL domain.
Activation of Notch signaling through cell-cell interactions (
Notch receptor and ligand expression in blood vessels
Notch1 is broadly expressed in many tissues, including the heart and vascular endothelial cells, while Notch4 expression is restricted to vascular endothelial cells [13–15], and Notch3 is predominantly expressed in vascular smooth muscle cells [16]. Transcriptional regulation of
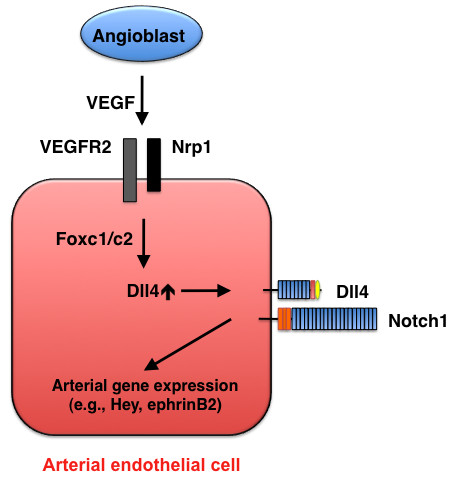
Figure 3
Figure 3 caption
Arterial cell specification mediated by Dll4-Notch signaling. During early development, VEGF (in concert with Foxc1/c2 transcription factors) induces Dll4 expression in endothelial cells, and Dll4-Notch signaling promotes arterial gene expression.
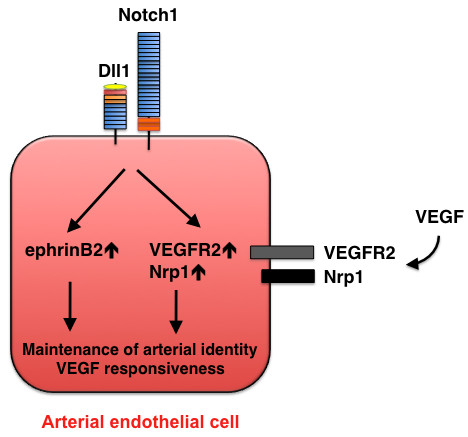
Figure 4
Figure 4 caption
Maintenance of arterial identity mediated by Dll1-Notch signaling. At a later stage of development, Dll1 expression is induced in arterial endothelial cells and is required for maintenance of the arterial phenotype. Dll1 also acts upstream of VEGF by regulating the expression of VEGFR2 and its co-receptor neuropilin 1 (Nrp1).
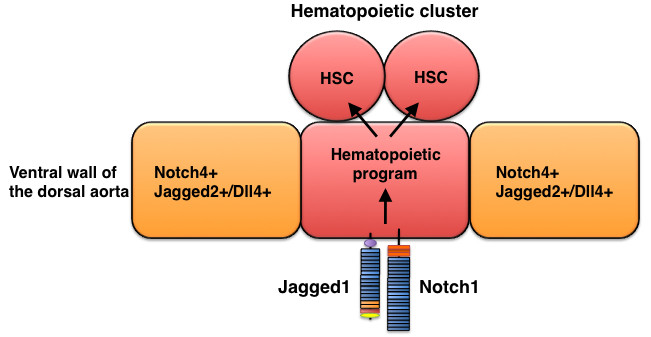
Figure 5
Figure 5 caption
Jagged1-mediated hematopoietic program in the dorsal aorta during development. Hematopoietic stem cells (HSC) descend from Jagged1+ Notch1+ endothelial cells in the aorta.
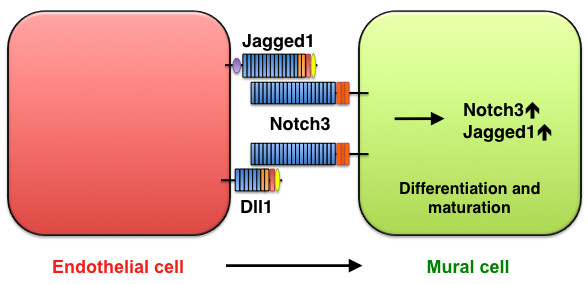
Figure 6
Figure 6 caption
Smooth-muscle maturation mediated by Jagged1/Dll1-Notch3 signaling. Jagged1 and Dll1 in endothelial cells activate Notch3 on mural cells, thereby promoting mural-cell maturation.
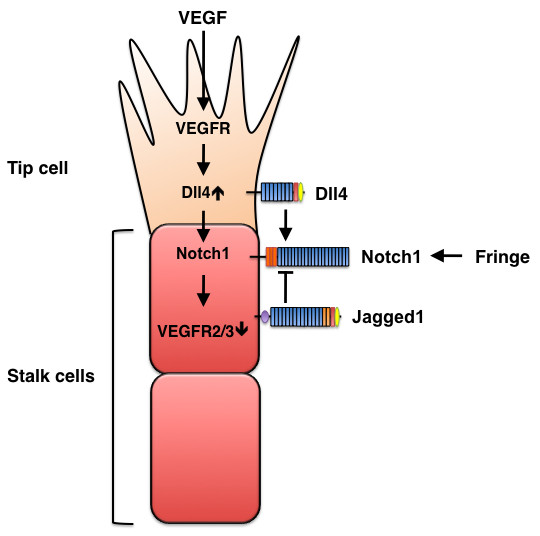
Figure 7
Figure 7 caption
Opposing effects of Dll4 and Jagged1 on sprouting angiogenesis. VEGF signaling induces Dll4 expression in tip cells, and Dll4, in turn, activates Notch signaling in stalk cells, which reduces stalk-cell sensitivity to VEGF stimulation and, consequently suppresses the tip-cell phenotype. Conversely, Jagged1 antagonizes Dll4-mediated Notch activation in stalk cells to increase tip cell numbers and enhances vessel sprouting. The antagonistic effects of the two ligands are controlled by Fringe-dependent modulation of Notch signaling.
Notch1, Notch4, and the ligands Dll1 and Dll4 during arterial specification and maintenance
Results from recent studies in zebrafish suggest that activation of Notch signaling by the Sonic hedgehog (Shh) and VEGF pathways is essential for arterial specification during development [29, 30]. Two Notch receptors, Notch1 and Notch4, are predominantly expressed in arterial endothelial cells of early mouse embryos.
Table 2
Mammalian Notch receptors and ligands involved in vascular development and disease
| Component | Phenotype/Role | References |
|---|---|---|
| Notch receptors | ||
| Notch1 | Proper vascular development; Postnatal neovascularization | [ |
| Notch3 | Maturation of vascular smooth muscle cells | [ |
| Notch4 | Null mice show normal vascular development; | [ |
| Notch ligands | ||
| Jagged1 | Dispensable for arterial specification; Formation of hematopoietic stem cells from the aorta; Smooth muscle differentiation and maturation; Proangiogenic regulation | [ |
| Dll1 | Maintenance of arterial identity; Arterial smooth muscle differentiation; Postnatal arteriogenesis | [ |
| Dll4 | Arterial specification; Tip cell and stalk cell selection during sprouting angiogenesis; Regulation of tumor angiogenesis | [ |
Of the four Notch ligands (Jagged1, Jagged2, Dll1, and Dll4) that are expressed in arterial endothelial cells, Dll4 alone is expressed in the dorsal aorta of mice at embryonic day 8.5 (E8.5), and its expression is restricted to vascular endothelial cells [13]; thus, Dll4 is believed to be the ligand for Notch1 and Notch4 during early vascular development (Figure 3).
Dll1 expression is detected in arterial endothelial cells at a later stage (E13.5) of mouse development [19] and continues to be restricted to arterial endothelial cells in adults [38]. Dll1 is not critically involved in arterial-cell specification; however, analyses in hypomorphic and endothelial-specific
Jagged1 does not play a critical role in arterial development [39–41] but is required for the definitive hematopoietic program in the dorsal aorta. After arterial and venous endothelial cells differentiate, the ventral region of the dorsal aorta, located in the aorta-gonad-mesonephros (AGM) region of the mid-gestation mouse embryo (around E10-11), generates the first adult hematopoietic stem cells (HSCs). Notch4 is broadly expressed throughout the aortic endothelium of the AGM, whereas Notch1 expression is restricted to the ventral region of the dorsal aorta [40, 42]. Importantly, three Notch ligands (Jagged1, Jagged2, and Dll4) have distinctive expression patterns in the dorsal aorta of the AGM: Jagged1 and Notch1 expression overlap in the dorsal aorta, Jagged2 expression occurs in endothelial cells adjacent to Notch1-positive endothelial cells, and Dll4 is expressed in both Notch1-positive and Notch1-negative endothelial cells [40, 42]. Analyses in
Notch3, Jagged1, and Dll1 during smooth-muscle differentiation and maturation
Notch3 is predominantly expressed in the vascular smooth muscle of arteries and is not expressed in veins. Mutations in human
Dll4 and Jagged1 in tip- and stalk-cell specification during sprouting angiogenesis
The formation of new blood vessels, a process known as angiogenesis, involves the sprouting of endothelial cells. In response to VEGF stimulation, filopodia extend from a migratory endothelial cell at the vessel's tip (i.e., the tip cell), and proliferative endothelial cells (i.e., stalk cells) form the trunk of the new vessel. Recent studies in mice and zebrafish clearly demonstrate that Notch signaling interacts with VEGF signaling during tip-cell and stalk-cell specification [5]. VEGF induces Dll4 expression in tip cells, then Dll4 activates the Notch pathway in adjacent endothelial cells to reduce expression of VEGFR2 and VEGFR3, thereby suppressing the tip-cell phenotype, and tip-cell phenotype suppression cell-autonomously promotes the stalk-cell phenotype. Together, these mechanisms balance tip-cell and stalk-cell selection and, consequently, limit the number of sprouting vessels (Figure 7). Genetic or pharmacological disruption of Dll4-Notch signaling leads to excessive tip-cell formation and vessel sprouting in cultured cells, in zebrafish and mouse embryos, and during tumor angiogenesis [23, 25, 46–51].
By using endothelial-specific
Notch ligands in pathological angiogenesis
Dll4 is expressed in tumor vasculature [26, 36, 53, 54], and as observed in studies of developmental angiogenesis, the blockade of Dll4-mediated Notch signaling (via systemic administration of Dll4-neutralizing antibodies [47, 48] and systemic or local administration of modified Dll4 proteins [47, 55]) increased tumor-vessel sprouting, which indicates that Dll4-Notch signaling is critical for tip- and stalk-cell selection during tumor angiogenesis. Remarkably, the inhibition of Dll4-Notch signaling increased neovascularization but impaired tumor growth, because the non-productive angiogenesis reduced tumor perfusion. Conversely, Dll4 activation of endothelial Notch signaling reduces tumor angiogenesis, but increases vessel diameter and perfusion, which enhances tumor growth [47, 56]. For these reasons, Dll4 is now recognized as a potential therapeutic target for tumor angiogenesis [57].
As described above, Jagged1 antagonizes Dll4 during sprouting angiogenesis [52], and overexpression of Jagged1 in tumor cells has been shown to enhance neovascularization and tumor growth [58]; however, the role of Jagged1 in pathological angiogenesis (including tumor angiogenesis) is not yet fully understood. Current findings suggest that angiogenic sprouting in the tumor is tightly controlled by positive and negative regulation of Jagged1 and Dll4 in both endothelial and non-endothelial cells. Recent studies have shown that a soluble form of Notch1 (Notch decoy) acts as an antagonist of ligand-dependent Notch signaling by (potentially) interfering with Dll1, Dll4, and Jagged1 [59, 60]. Importantly, the Notch decoy reduces tumor growth without increasing vessel growth, which suggests that the effects of the Notch decoy differ from those induced by Dll4 blockade. It is therefore likely that the proangiogenic function of Jagged1 in tumor cells and endothelial cells could also influence tumor angiogenesis.
Notch signaling in peripheral ischemia
Notch signaling is also required for angiogenesis in peripheral ischemia models [32, 38] (Table 2). Blood flow recovery and postnatal neovascularization in response to hind-limb ischemia are impaired in both global and endothelial-specific
Concluding remarks and future perspectives
Studies performed in the past few years clearly demonstrate that the different Notch ligands have distinct functions in vascular development and disease. This understanding has prompted numerous investigations into the mechanisms by which Notch signaling is essential for multiple aspects of vascular biology. However, given that the effects of Notch pathway activation on endothelial cells are context-dependent [4], many questions remain to be answered. First, the upstream signaling pathways that control the expression of Notch ligands in blood vessels remain largely unknown; VEGF induces Dll4 expression in endothelial cells (Table 1), but Jagged1 is absent in tip cells where Dll4 is highly expressed, which suggests that the two ligands are regulated differently. Second, the selective activation of Notch in vascular endothelium remains unclear; for example, Notch signaling is not activated in arteries of
Author's information
The author is an Associate Professor at Northwestern University School of Medicine, USA. He completed his postdoctoral training in the lab of Brigid Hogan at the Howard Hughes Medical Institute at Vanderbilt University Medical Center, USA. He graduated with a Ph.D. in Molecular and Cellular Biology from the University of Tokyo, Japan.
Acknowledgements
The author thanks W. Kevin Meisner, PhD, ELS, for editorial support. This work was supported by a NIH grant (RO1 HL074121) to TK.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2
Authors’ original file for figure 3
Authors’ original file for figure 4
Authors’ original file for figure 5
Authors’ original file for figure 6
Authors’ original file for figure 7
References
- Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678-689.
- Notch signaling in vascular morphogenesis. Curr Opin Hematol. 2004;11:278-283.
- Notch signaling in vascular development and physiology. Development. 2007;134:2709-2718.
- Notch signaling in blood vessels: who is talking to whom about what?. Circ Res. 2007;100:1556-1568.
- Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196-208.
- Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21:2511-2524.
- Notch signaling and diseases: an evolutionary journey from a simple beginning to complex outcomes. Biochim Biophys Acta. 2008;1782:489-497.
- OSM-11 facilitates LIN-12 Notch signaling during Caenorhabditis elegans vulval development. PLoS Biol. 2008;6:e196-.
- The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216-233.
- Cell and molecular biology of Notch. J Endocrinol. 2007;194:459-474.
- ADAM proteases: ligand processing and modulation of the Notch pathway. Cell Mol Life Sci. 2008;65:2056-2068.
- The many facets of Notch ligands. Oncogene. 2008;27:5148-5167.
- Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343-1352.
- Glucocorticoid and growth factor synergism requirement for Notch4 chromatin domain activation. Mol Cell Biol. 2007;27:2411-2422.
- Molecular determinants of NOTCH4 transcription in vascular endothelium. Mol Cell Biol. 2005;25:1458-1474.
- The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest. 2000;105:597-605.
- Bare rudiments of notch signaling: how receptor levels are regulated. Trends Biochem Sci. 2007;32:477-485.
- Expression of the mouse Delta1 gene during organogenesis and fetal development. Mech Dev. 1999;84:165-168.
- DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood. 2009;113:5680-5688.
- Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev. 2001;108:161-164.
- NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ Res. 2009;104:466-475.
- Foxc transcription factors directly regulate Dll4 and Hey2 expression by interacting with the VEGF-Notch signaling pathways in endothelial cells. PLoS ONE. 2008;3:e2401-.
- Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776-780.
- Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14-25.
- Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci. 2007;104:3219-3224.
- Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Cancer Res. 2005;65:8690-8697.
- The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol. 2006;294:458-470.
- Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931-939.
- Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675-3683.
- Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127-136.
- Essential role of endothelial Notch1 in angiogenesis. Circulation. 2005;111:1826-1832.
- Critical role of endothelial Notch1 signaling in postnatal angiogenesis. Circ Res. 2007;100:70-78.
- Vascular patterning defects associated with expression of activated Notch4 in embryonic endothelium. Proc Natl Acad Sci. 2001;98:5643-5648.
- Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc Natl Acad Sci. 2005;102:9884-9889.
- Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 2004;18:2474-2478.
- Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci. 2004;101:15949-15954.
- Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469-2473.
- Notch ligand Delta-like 1 is essential for postnatal arteriogenesis. Circ Res. 2007;100:363-371.
- Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc Natl Acad Sci. 2008;105:1955-1959.
- Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. EMBO J. 2008;27:1886-1895.
- Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet. 1999;8:723-730.
- RBPjkappa-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development. 2005;132:1117-1126.
- Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707-710.
- Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev. 2004;18:2730-2735.
- Determinants of Notch-3 receptor expression and signaling in vascular smooth muscle cells: implications in cell-cycle regulation. Circ Res. 2002;91:999-1006.
- Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839-844.
- Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032-1037.
- Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083-1087.
- Cell-autonomous notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. FASEB J. 2005;19:1027-1029.
- Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781-784.
- The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci. 2007;104:3225-3230.
- The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124-1135.
- The role of the vascular endothelial growth factor-Delta-like 4 ligand/Notch4-ephrin B2 cascade in tumor vessel remodeling and endothelial cell functions. Cancer Res. 2006;66:8501-8510.
- Delta4, an endothelial specific notch ligand expressed at sites of physiological and tumor angiogenesis. Differentiation. 2001;69:135-144.
- Inhibition of Dll4-mediated signaling induces proliferation of immature vessels and results in poor tissue perfusion. Blood. 2007;109:4753-4760.
- Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007;67:11244-11253.
- The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer. 2007;7:327-331.
- Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer Cell. 2005;8:13-23.
- Notch signaling regulates tumor angiogenesis by diverse mechanisms. Oncogene. 2008;27:5132-5137.
- A notch1 ectodomain construct inhibits endothelial notch signaling, tumor growth, and angiogenesis. Cancer Res. 2008;68:4727-4735.
- Microfibrillar proteins MAGP-1 and MAGP-2 induce Notch1 extracellular domain dissociation and receptor activation. J Biol Chem. 2006;281:10089-10097.
- The extracellular matrix protein MAGP-2 interacts with Jagged1 and induces its shedding from the cell surface. J Biol Chem. 2005;280:20349-20355.
- Microfibril-associate glycoprotein-2 (MAGP-2) promotes angiogenic cell sprouting by blocking notch signaling in endothelial cells. Microvasc Res. 2008;76:7-14.
- Transcriptome analysis of endothelial cell gene expression induced by growth on matrigel matrices: identification and characterization of MAGP-2 and lumican as novel regulators of angiogenesis. Angiogenesis. 2007;10:197-216.
- DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5:168-177.

