A role for Egfl7 during endothelial organization in the embryoid body model system
Journal of Angiogenesis Research. 2010;
Received: 12 November 2009 | Accepted: 19 February 2010 | Published: 19 February 2010
Vascular Cell ISSN: 2045-824X
Abstract
Epidermal growth factor-like domain 7,
Background
Epidermal growth factor-like domain 7,
We chose the embryoid body (EB) differentiation model to examine the effect of
Methods
Knock-down construct and siRNA production
The siRNA sequences used for
Production of lentivirus and stable embryonic stem cell knock-down clones
HEK 293T cells were co-transfected with FG12 lentiviral vectors carrying the siRNA sequence, HIV-1 lentiviral packaging constructs (pMDLg/pRRE and pRSV-REV), and pVSV-G (a plasmid coding for the G protein of the vesicular stomatitis virus) by the calcium phosphate method. Virus supernatant was collected 24-40 h after transfection and concentrated by ultracentrifugation (22,000 × g). The virus titers were determined on 3T3 cells by counting the number of eGFP+ cells under a microscope, and were 5 × 106-1.5 × 108 infectious units/ml. Mouse ESCs (W4/129S6; Taconic) were grown on a feeder layer of irradiated mouse embryonic fibroblasts (MEFs) in DMEM supplemented with 15% FBS, 20 mM HEPES, 0.1 mM non-essential amino acids, 0.1 mM β-mercaptoethanol, 100 U/ml penicillin/streptomycin, 0.3 mg/ml L-glutamine, and 103U/ml LIF (ESGRO; Chemicon). ESCs were infected in the presence of 8 μg/ml polybrene at a MOI of 1-2. Individual eGFP+ clones were isolated and assessed for expression of the knock-down construct by RT-PCR and western blot. RNA and protein was extracted from cells using the PARIS kit (Ambion) as per manufacturer's instructions. Reverse transcription was carried out using the SuperScript III First-Strand Synthesis System (Invitrogen), followed by PCR using the following primers;
Real-time PCR analysis of Egfl7 and miR-126 levels
For real-time PCR analysis, total RNA was isolated from ESCs using the RNAqueous-Micro Kit (Ambion) as per manufacturer's instructions.
Embryonic stem cell growth rate
ESC growth rates were determined essentially as described by Udy
Embryonic stem cell differentiation as embryoid bodies
ESCs were MEF-depleted and seeded in 30 μl hanging drops at 8 × 104 cells/ml in differentiation medium (as for ES cell media, except 20% FBS and no LIF). Two days later EBs were grown in suspension, and then harvested at either 7 or 14 days after initial seeding. Where TGF-β was used, 2.5 ng/ml recombinant human TGF-β (R&D Systems) was added to medium prior to making hanging drops, and also for subsequent feeding. In other experiments, conditioned medium from wild-type, or scrambled control, EBs was used on knock-down clones during differentiation to day 7 EBs. EBs were fixed in 4% PFA followed by 10% and 20% sucrose, and frozen in a 1:1 solution of OCT: 30% sucrose. 12 μm sections were used for indirect immunofluorescence (IF) staining.
Collagen type I sprouting angiogenesis assay
Ten individual 7 or 14 day EBs were plated onto 1.5 ml of solidified collagen type I medium in a 35 mm diameter plate and allowed to settle overnight in differentiation medium, before a second collagen layer was added. The collagen medium was made as described by Feraud
Indirect immunofluorescence staining
EB cryosections were fixed in ice-cold acetone, or methanol (for Flk1 staining), and EBs within collagen type I gels were fixed in 4% PFA. Samples were blocked with 10% normal donkey serum and 5% non-fat dried milk, and antibodies were diluted in 5% non-fat dried milk. Collagen gels were also permeabilized using 0.5% triton X-100. For Annexin-V staining 2% BSA was used instead of milk. Sequential double-staining was carried out with the anti-CD31 antibody first, and antibodies were used as follows; rat anti-mouse CD31, 5 μg/ml (BD Biosciences), goat anti-mouse Flk1, 4 μg/ml (Santa Cruz), rabbit anti-mouse Collagen IV, 5 μg/ml (Chemicon), goat anti-mouse VE-Cadherin, 5 μg/ml (R&D Systems), rabbit anti-mouse Claudin-5, 2.5 μg/ml (Invitrogen), rabbit anti-mouse Ki67, 1.5 μg/ml (Abcam), rabbit anti-mouse Annexin-V, 2.5 μg/ml (Abcam). Rat, rabbit, and goat IgG controls were used on adjacent sections. Signals were detected with donkey anti-rat IgG conjugated with Cy3 or Cy5, and donkey anti-rabbit or -goat IgG conjugated with Cy5 or Cy3 (Jackson ImmunoResearch). Cryosections were mounted in ProLong Gold Antifade reagent with DAPI (Invitrogen). Collagen gels were incubated with Hoechst 33342 nuclear dye (Invitrogen) and mounted in Vectashield hard-set mounting medium (Vector Labs). Images were taken using an Axioplan 2 imaging microscope (Carl Zeiss), or a Leica TSC SP2 confocal laser microscope (Leica Microsystems).
Quantification of vascular structures
For quantification of EB cryosections and collagen gel-embedded EBs, image acquisition was performed with an ORCA-ER black and white camera (Hamamatsu Photonics) driven by Openlab software (Improvision, Ltd.). Relative CD31+, Ki67+, and DAPI+, areas were measured by determining the number of pixels corresponding to the fluorescent signal using the 'magic wand tool' in Photoshop (Adobe Systems Inc.). Individual EBs were also scored for the presence of CD31+ 'cords', 'sheets', or both. Cords were defined as CD31+ structures of more than two cells in length, and not more than two cells in width, determined by counting DAPI-stained nuclei in overlaid images. CD31+ sheet structures were defined as being more than four cells in diameter, and more than four cell's distance from another sheet to be counted individually. CD31+ sheets were also analyzed separately, and the Ki67+ pixels within each sheet determined. Where indicated, confocal images were captured using Leica Confocal Software. All analysis for the collagen gel-embedded EBs was done using Photoshop. Relative CD31+ sprout length was determined using the 'measure' tool and branching points were defined as where two or more CD31+ sprouts radiated from. All statistical analysis was carried out using Prism4 (GraphPad Software, Inc.).
Results and Discussion
Lentiviral-mediated knock-down of Egfl7
The FG12 lentiviral vector [14, 18] was used for delivery of siRNAs into ESCs. This vector has an RNA polymerase III promoter (H1) to drive siRNA expression, and an UbiC promoter to drive marker gene (eGFP) expression (Figure 1a). The use of RNA polymerase III and UbiC promoters is an established technique in lentiviral-mediated knock-down [14, 19]. The eGFP expression serves as a reliable marker to demonstrate the presence and expression of the
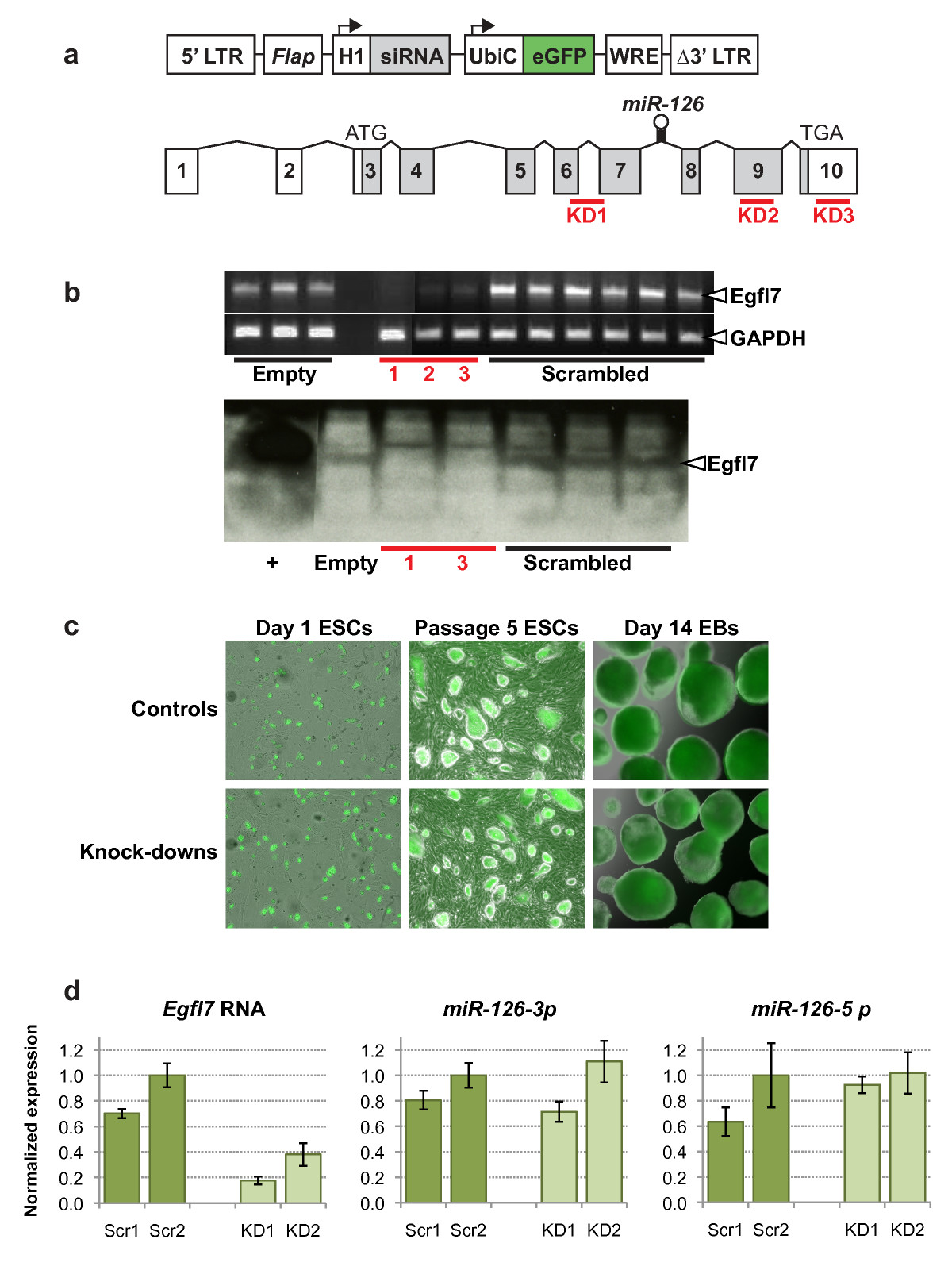
Figure 1
Figure 1 caption
Knock-down ofexpression in mouse embryonic stem cells. (a) Lentiviral construct used for siRNA-mediated knock-down of (top), and gene structure (bottom) showing non-coding (unshaded) and coding (shaded) exons. The three siRNA target sequences are shown as red bars, and the location of the microRNA is shown within intron 7. (LTR, long terminal repeat; , DNA flap; H1, human H1 RNA pol III promoter; UbiC, human ubiquitin c promoter; eGFP, enhanced green fluorescent protein; WRE, woodchuck response element). (b) Verification of knock-down in mouse ESCs by RT-PCR (top) and western blot (bottom). (Empty, lentiviral construct alone; 1, 2, 3 (corresponding to KD1, KD2, KD3), lentivirus containing siRNA sequence; scrambled, lentivirus containing scrambled siRNA control sequence; +, HEK293 cells transfected with a His-tagged vector). (c) Endogenous eGFP expression in lentivirus infected undifferentiated ESCs 8 hours after plating (left two panels) and after passaging 5 times (middle two panels), and in ESC-derived EBs after 14 days of differentiation (right two panels). Magnification used 69×. (d) Quantitative PCR was carried out for and the microRNAs and . Expression was normalized to (for ) or (for the microRNAs), and is shown relative to a value of 1.0 for the scrambled control 2 (Scr2).
Egfl7 knock-down reduces embryonic stem cell growth rate
To determine whether
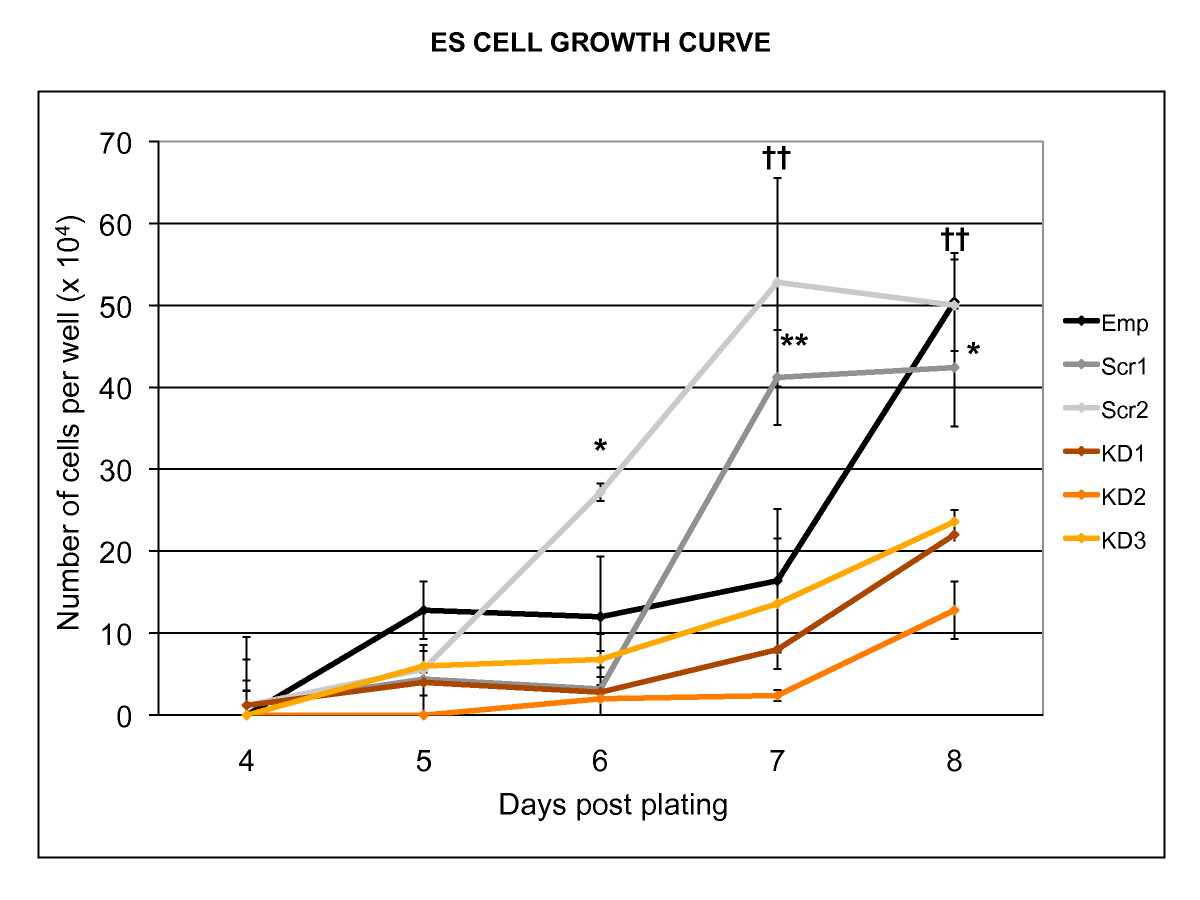
Figure 2
Figure 2 caption
Effect ofknock-down on embryonic stem cell growth rate. Knock-down of resulted in a reduced growth rate in undifferentiated mouse ESCs. The experiment was carried out three times, yielding similar results. (* p < 0.05, ** p < 0.01, †† p < 0.001; Emp, empty lentiviral construct; Scr1, Scr2, Scrambled siRNA sequences; KD1, KD2, KD3, Knock-down siRNA sequences).
Abnormal endothelial sheets form in Egfl7 knock-down embryoid bodies
EBs were formed from the three knock-down and three control ESC clones by the hanging drop method [20].
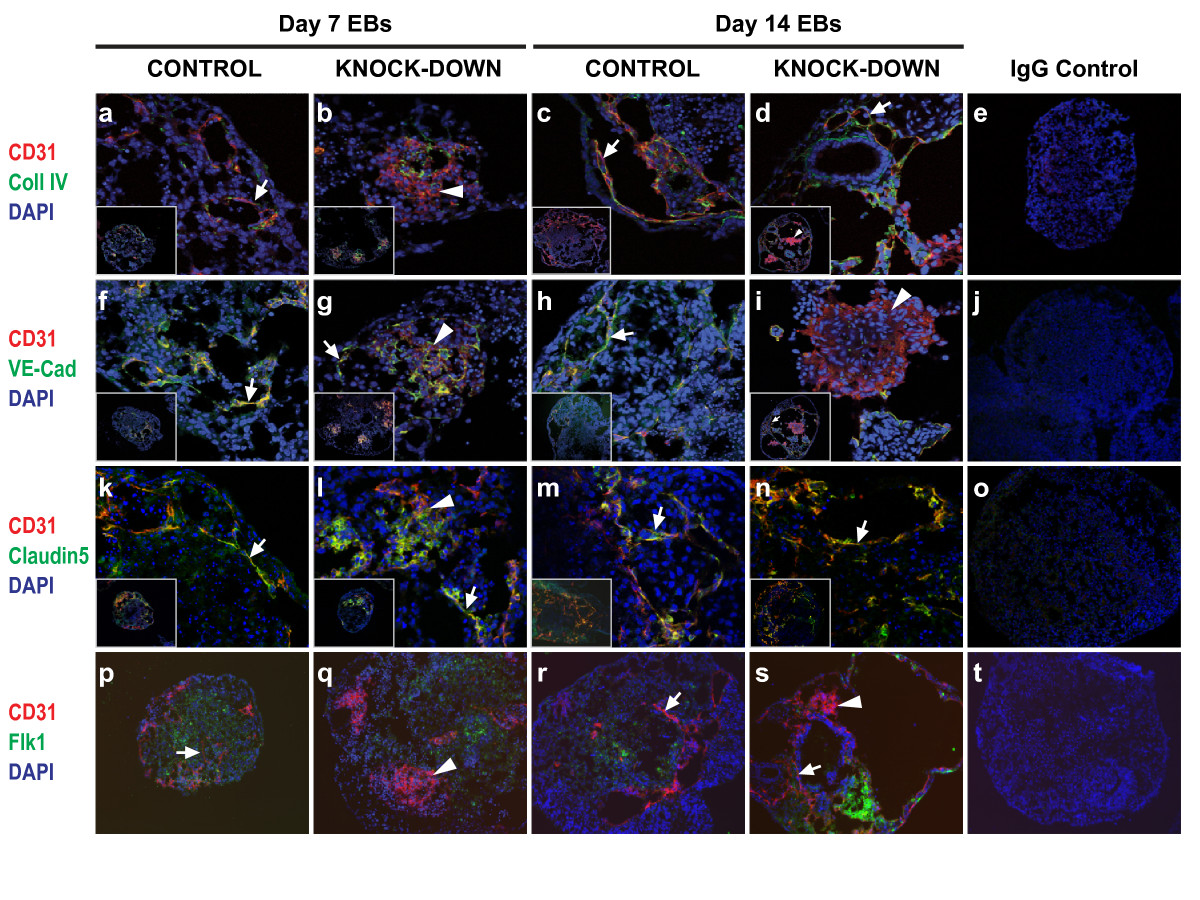
Figure 3
Figure 3 caption
Effect ofknock-down onvitro endothelial development. Cryosections of EBs at day 7 (a, b, f, g, k, l, p, q) and day 14 (c, d, h, i, m, n, r, s) were subjected to indirect IF using antibodies against CD31 plus collagen IV (a-d), CD31 plus VE-cadherin (f-i), CD31 plus claudin 5 (k-n), or CD31 plus Flk1 (p-s). Magnification used; (a-d, f-i, k-n) inserts show whole EBs at 20×, and large panels show detail at 63×, (p-s) panels show EBs at 20×, (e, j, o, t) panels show whole EB IgG controls at 20× (arrows, CD31+ cords; arrowheads, CD31+ sheets). Images were acquired using a confocal laser microscope (Leica Microsystems; a-o) or an Axioplan 2 imaging microscope (Carl Zeiss; p-t).
Vascular structures within EBs were analyzed at 7 days and 14 days of differentiation, as these time points correspond approximately with early organogenesis and later remodelling stages respectively [13]. Sections through EBs revealed the presence of two clearly distinguishable CD31+ cell structures, which we describe here as 'cords' (Figure 3; arrows) and 'sheets' (Figure 3; arrowheads). 'Cords' are defined as more than two CD31+ cells in length and a maximum of two CD31+ cells in width, and 'sheets' as aggregates of more than four CD31+ cells in length and width. These structures were viewed on 2-dimensional cryosections through EBs. Due to the spherical shape of EBs it is probable that the 'cords' are part of a larger network existing in multiple axes within the EBs, and that the 'sheets' represent one plane within 3-dimensional clusters of CD31+ cells. Similar CD31+ 'sheets' have been described in EBs derived from laminin γ1-deficient ESCs [12]. To further characterize the CD31+ structures present, EB sections were co-stained with antibodies against other endothelial markers. Collagen IV is a major constituent of the basement membrane, and its deposition is characteristic of normal blood vessel formation and is required for subsequent angiogenesis [12, 21, 22]. Vascular endothelial (VE)-cadherin is the major transmembrane component of adherens junctions, and sustains cell-cell recognition and adhesion [23]. Used together with CD31, which is expressed on haematopoietic cells as well as ECs, VE-cadherin is considered to be the gold standard for EC-specific markers [24]. Claudin-5 is an endothelial-specific component of tight-junctions, which control para-cellular permeability and polarity [25, 26]. Flk1, a VEGF-A receptor, is an early marker of hematopoietic and endothelial cells [27].
At the day 7 time point CD31+ cords which co-expressed collagen IV, VE-cadherin, and claudin 5 were seen in all of the control and knock-down clones (Figures 3a, f, g, k, and 3l; arrows). However, the presence of large aggregates of CD31+ cells clearly distinguished the knock-down clones from the controls (Figures 3b, g, l, and 3q; arrowheads). By day 14 of differentiation more extensive endothelial cord networks had formed in all of the clones (Figures 3c, d, h, i (inset), 3m, n, r, and 3s; arrows), and some sheets were still present in the knock-down EBs (Figures 3d (inset), 3i, and 3s). Quantification of the CD31+ structures revealed a striking difference between controls and knock-downs. At day 7 the majority of the control EBs contained cords, whereas most of the knock-downs contained sheets as well as cords (Figure 4a). The spatial organization of ECs within these sheets were reminiscent of the midline angioblast aggregates seen in
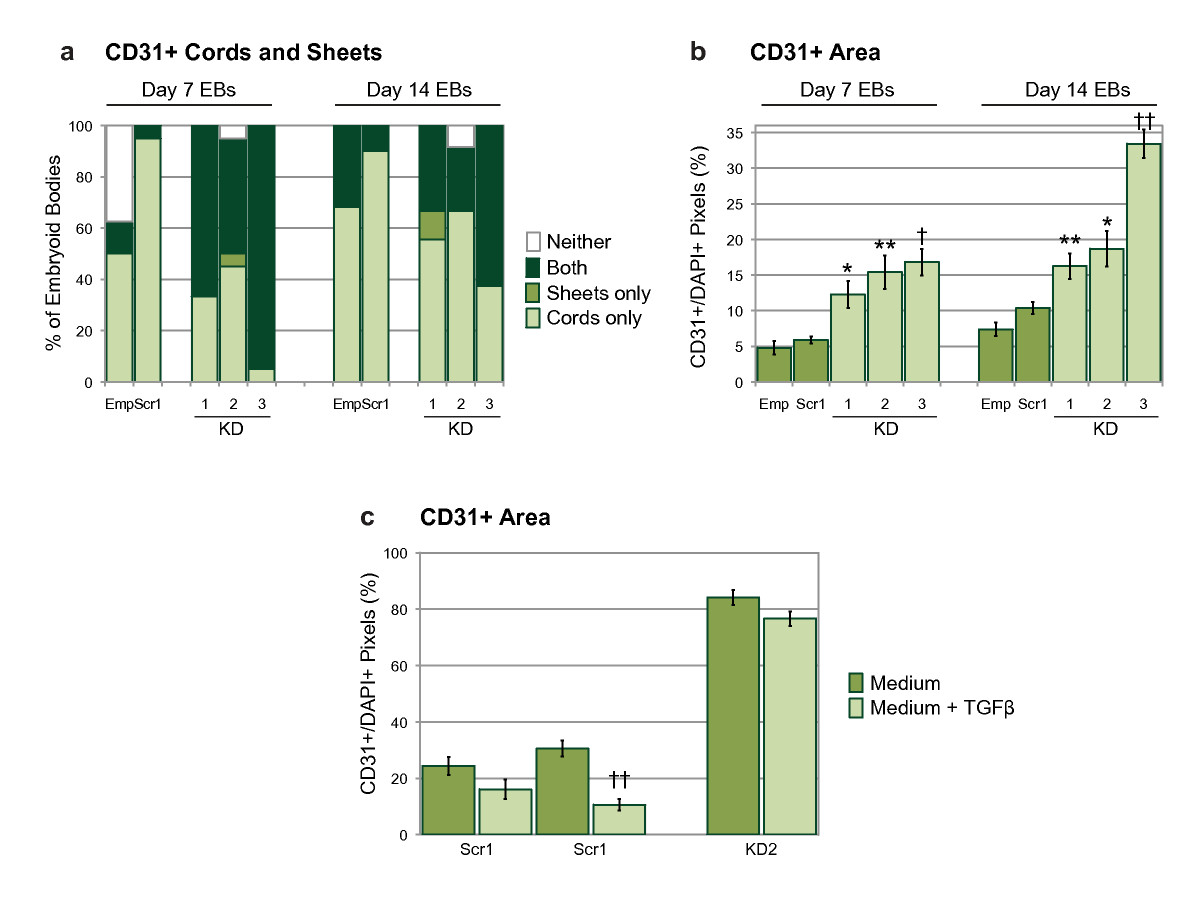
Figure 4
Figure 4 caption
Quantification of CD31+ structures within embryoid bodies. (a) Percentage of EBs containing CD31+ cords only, sheets only, both cords and sheets, or neither structure, as defined in Methods. (b) Relative CD31+ area on EB sections, measured in pixels and shown as a ratio of DAPI+ pixel number. Statistical significance was determined by one-way ANOVA followed by a Bonferroni post-test. (c) Relative CD31+ area on EBs sections; EBs grown +/- TGF-β. Statistical significance was determined by two-tailed, unpaired, t-tests. Bars are means ± S.E.M. (Emp, empty lentiviral construct; Scr1, scrambled siRNA sequence; KD1-3, knock-down siRNA sequences; = 16-36 for controls, = 20-29 for knock-downs; * p < 0.05, ** p < 0.01, † p < 0.005, †† p < 0.001). The data shows results from one experiment, which was carried out twice with similar results.
When conditioned medium from wild-type, or scrambled control, EBs was added to cultures of knock-down ESCs during differentiation to day 7 EBs, we did not observe a rescue of the mutant phenotype (data not shown). This suggests that Egfl7 may act in a cell-autonomous manner. It has recently been shown that inhibition of the anti-proliferative transforming growth factor beta (TGF-β) during
CD31+ sheets show increased cell proliferation
To address whether over-proliferation or reduced apoptosis of CD31+ cells contributed to the formation of the abnormal aggregates in knock-down clones, EB sections were co-stained with antibodies against Ki67 and Annexin V. The nuclear protein Ki67 is a marker for proliferation, and Annexin V is visible only in apoptotic and necrotic cells [29, 30]. At the 7 day time point there were many Ki67+ cells in the control EBs, whereas the knock-downs contained fewer of these cells, indicating reduced proliferation compared with the controls (Figures 5a and 5b; Figure 6a). At the 14 day time point all of the EBs, regardless of genotype, contained fewer Ki67+ cells compared with the 7 day EBs (Figures 5c and 5d; Figure 6a). In contrast, the number of cells expressing Annexin V increased over time, but did not differ between the controls and knock-downs (Figures 5f, g, h, and 5i). Despite a significant reduction in proliferation compared with controls (Figure 6a), all of the day 7 knock-down clones showed at least equal, if not higher, levels of proliferation within CD31+ sheets compared with the whole EB area (Figure 6b). This suggests that while knock-down of Egfl7 resulted in reduced proliferation, the abnormal CD31+ sheet areas proliferated as much as, if not more than, the rest of the EB structure. Since Egfl7 knock-down did not affect Annexin V levels (Figures 5f, g, h, i, and 5j), this suggests that the CD31+ sheets were not the result of localized cell death. It is therefore likely that these sheets occur, at least in part, due to over-proliferation of CD31+ cells in the knock-down clones. At the later time point the number of CD31+ sheets was markedly reduced, and those that existed contained a much lower proportion of proliferative cells than in the entire EB. Since knock-down of
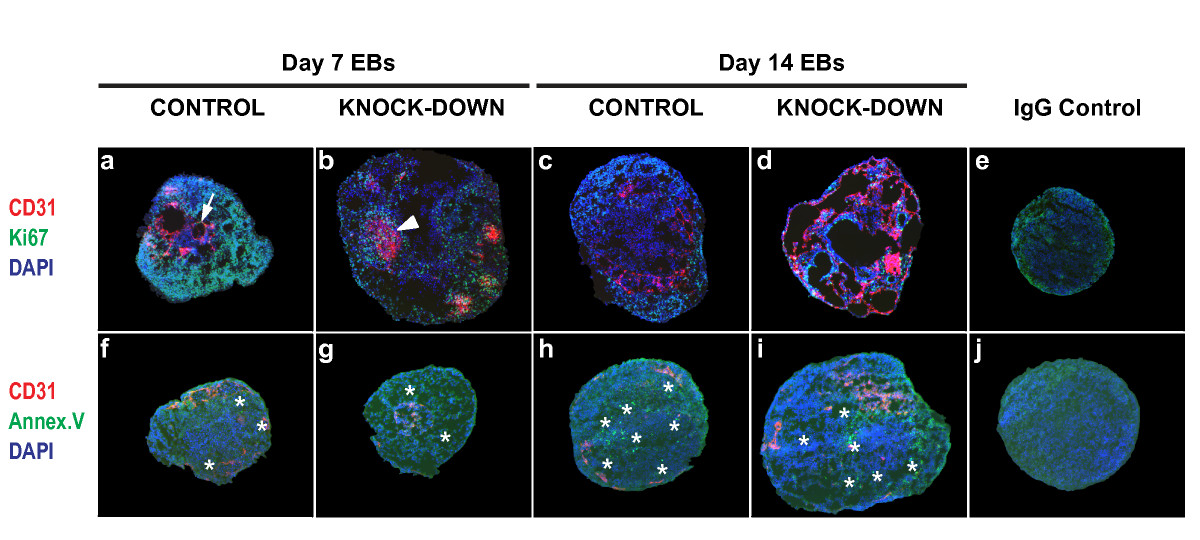
Figure 5
Figure 5 caption
Effect ofknock-down on embryoid body proliferation and apoptosis. Cryosections of EBs differentiated for 7 or 14 days were subjected to indirect IF using antibodies against CD31 plus Ki67 (40× magnification used; upper panels, a-d), or CD31 plus Annexin V (20× magnification used; bottom panels, f-i), (arrow, CD31+ cord; arrowhead, CD31+ sheet; asterisks, Annexin V+ cells). IgG controls are shown at 40× and 20× magnification (e, j). Images were acquired using an Axioplan 2 imaging microscope (Carl Zeiss).
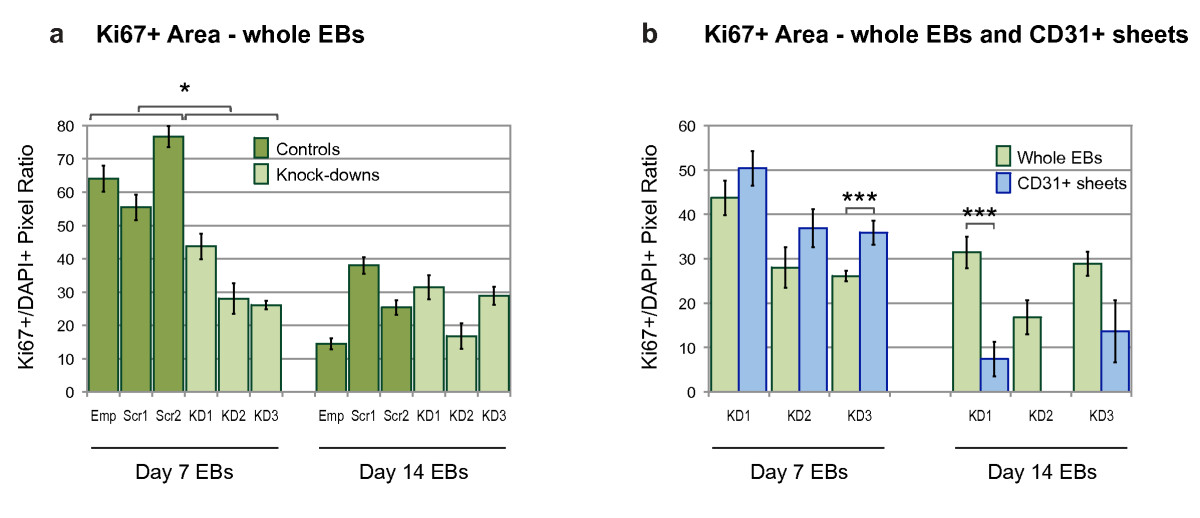
Figure 6
Figure 6 caption
Quantification of Ki67+ cells within embryoid bodies. The effect of knock-down on proliferation was quantified in day 7 and day 14 EBs using an antibody against Ki67. Relative Ki67+ area was measured in pixels and shown as a ratio of DAPI+ pixel number. (a) Whole EB sections were compared between control and knock-down clones at each time point ( = 10-16 for controls, = 9-23 for knock-downs). (b) Relative Ki67+ area of whole EBs, and CD31+ sheet areas, in knock-down EB clones. Statistical significance was determined by two-tailed, unpaired, t-tests. Bars are means ± S.E.M (Emp, empty lentiviral construct; Scr1, Scr2, scrambled siRNA sequences; KD1-3, knock-down siRNA sequences; = 9-23 for whole EBs, = 1-47 for CD31+ sheets; * p < 0.05, ** p < 0.01, † p < 0.005, †† p < 0.001).
Egfl7 knock-down is associated with endothelial sheet formation during sprouting angiogenesis
We next examined the effect of
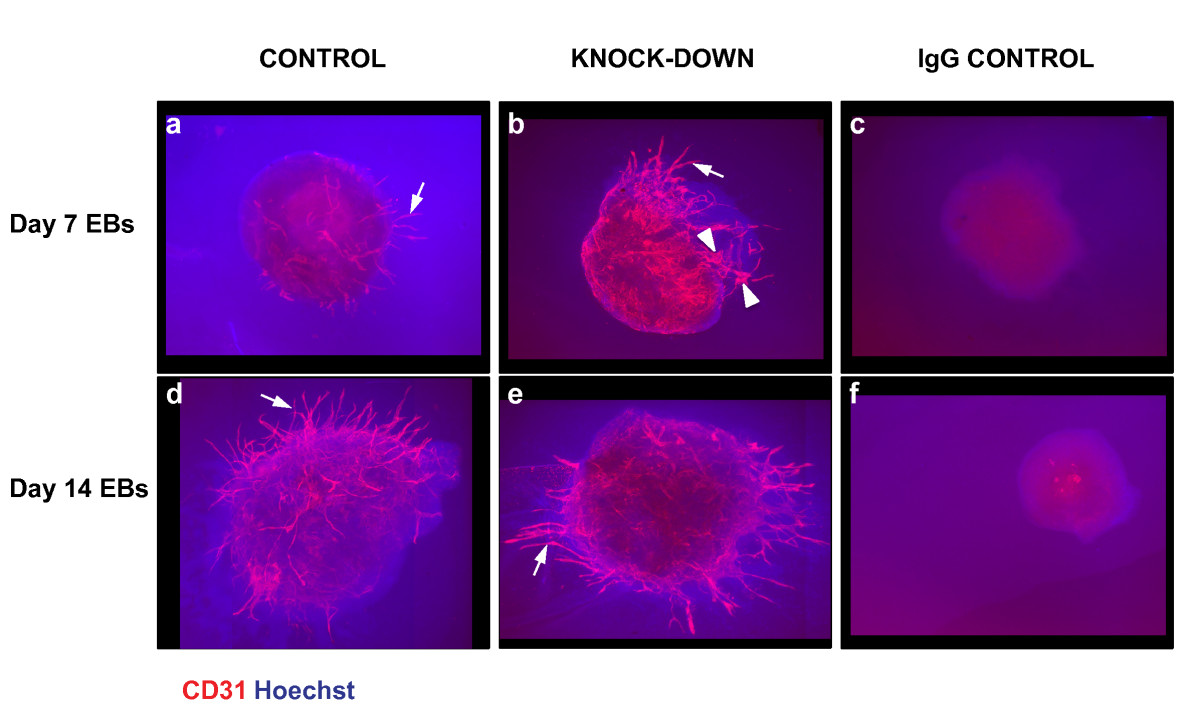
Figure 7
Figure 7 caption
Effect ofknock-down on embryoid body sprouting angiogenesis. Day 7 and day 14 differentiated EBs were grown between two layers of collagen type I gel for 11 days, before being subjected to indirect IF using an antibody against CD31, and Hoechst nuclear dye (arrows, CD31+ cords; arrowheads, CD31+ sheets).
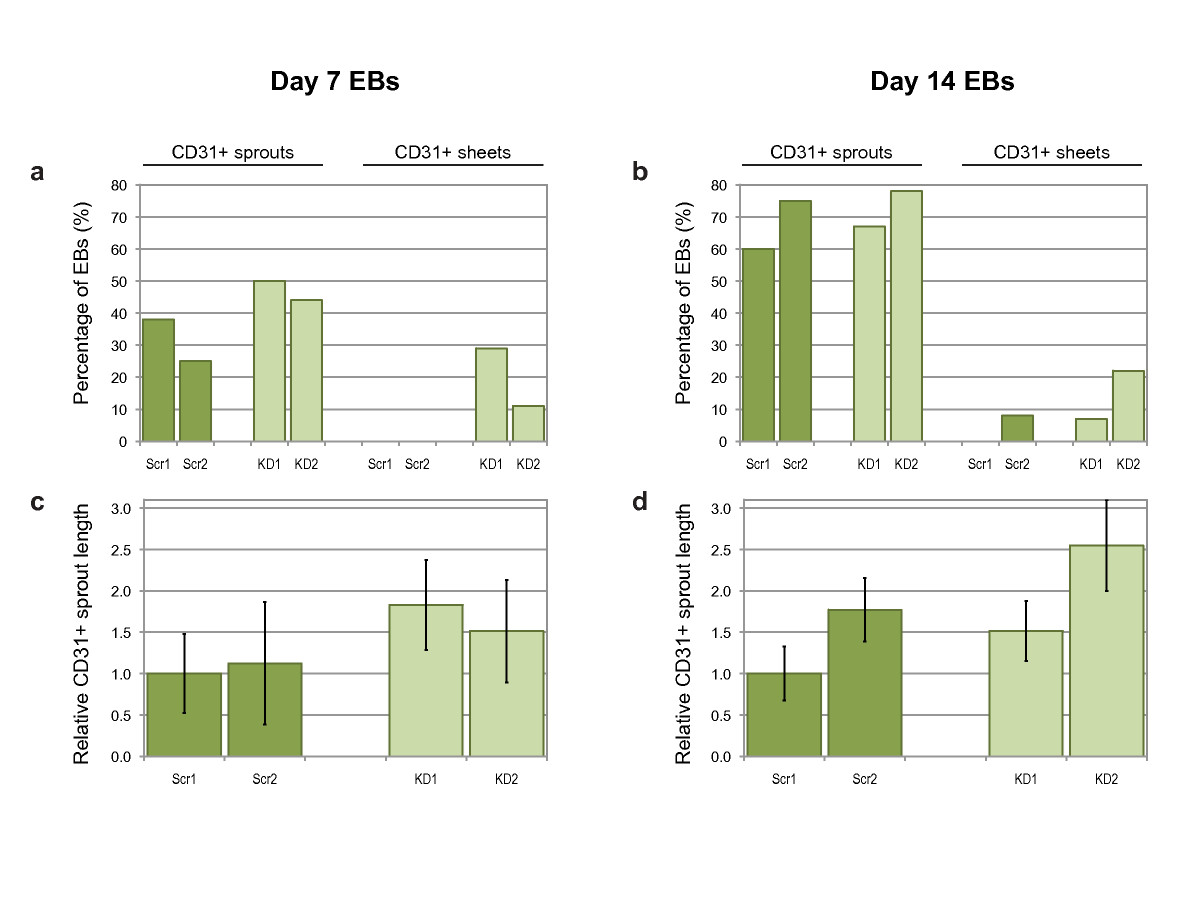
Figure 8
Figure 8 caption
Quantification of CD31+ sprouting angiogenesis. Percentage of day 7 (a) or day 14 (b) EBs with either CD31+ sprouts or CD31+ sheets. Average CD31+ sprout length from day 7 (c) or day 14 (d) EBs, normalized to Scr1 values. Bars are means ± S.E.M. (Scr1 and Scr2, scrambled siRNA sequences; KD1 and KD2, knock-down siRNA sequences; = 10 and 12 for controls, = 15 and 9 for knockdowns).
Recent work by Kuhnert
In conclusion, our results suggest that
Acknowledgements
We would like to thank Drs. Jan Kitajewski and Carrie Shawber at Columbia University Medical School and members of the Stuhlmann lab for helpful discussions on the project. We also thank the Molecular Cytology Core Facility at Memorial Sloan-Kettering Cancer Center for help with the confocal microscope. We thank Drs. Xiao-Feng Qin and David Baltimore (Caltech, CA) for providing us with the FG12 lentivirus vector. Funding for this work was provided in part by an American Heart Association fellowship 0525046Y to AD, and by a National Institutes of Health grant RO1 HL082098 to HS.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2
Authors’ original file for figure 3
Authors’ original file for figure 4
Authors’ original file for figure 5
Authors’ original file for figure 6
Authors’ original file for figure 7
Authors’ original file for figure 8
References
- Egfl7, a novel epidermal growth factor-domain gene expressed in endothelial cells. Dev Dyn. 2004;230:316-324.
- EGFL7 Is a Chemoattractant for Endothelial Cells and Is Up-Regulated in Angiogenesis and Arterial Injury. Am J Pathol. 2005;167:275-284.
- Expression of EGFL7 in primordial germ cells and in adult ovaries and testes. Gene Expression Patterns. 2008;8:389-396.
- The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428:754-758.
- Egfl7 knockdown causes defects in the extension and junctional arrangements of endothelial cells during zebrafish vasculogenesis. Developmental Dynamics. 2008;237:580-591.
- EGFL7 regulates the collective migration of endothelial cells by restricting their spatial distribution. Development. 2007;134:2913-2923.
- Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135:3989-3993.
- Methods for inducing embryoid body formation: In vitro differentiation system of embryonic stem cells. Journal of Bioscience and Bioengineering. 2007;103:389-398.
- In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol. 1995;7:862-869.
- A complex linkage in the developmental pathway of endothelial and hematopoietic cells. Current Opinion in Cell Biology. 2001;13:673-678.
- Embryonic stem cell-derived neurogenesis - Retinoic acid induction and lineage selection of neuronal cells. Cell and Tissue Research. 2001;305:171-176.
- Laminin deposition is dispensable for vasculogenesis but regulates blood vessel diameter independent of flow. Faseb Journal. 2008;22:1530-1539.
- Use of developmental marker genes to define temporal and spatial patterns of differentiation during embryoid body formation. J Exp Zool. 1999;284:67-81.
- Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci USA. 2003;100:183-188.
- ES cell cycle rates affect gene targeting frequencies. Experimental Cell Research. 1997;231:296-301.
- Vasculogenesis and angiogenesis from in vitro differentiation of mouse embryonic stem cells. Methods Enzymol. 2003;365:214-228.
- Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood. 1996;88:3424-3431.
- Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868-872.
- Peroxisome proliferator-activated receptor gamma overexpression and knockdown: impact on human B cell lymphoma proliferation and survival. Cancer Immunology Immunotherapy. 2009;58:1071-1083.
- In vitro differentiation of mouse ES cells: bone and cartilage. Methods Enzymol. 2003;365:251-268.
- Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619-1628.
- Functional analysis of the cytoplasmic domain of the integrin alpha 1 subunit in endothelial cells. Blood. 2008;112:3242-3254.
- Endothelial adherens junctions: Implications in the control of vascular permeability and angiogenesis. Journal of Clinical Investigation. 1996;98:1949-1953.
- Lentiviral rescue of vascular endothelial growth factor receptor-2 expression in Flk1-/- embryonic stem cells shows early priming of endothelial precursors. Stem Cells. 2007;25:2987-2995.
- Endothelial claudin: Claudin-5/TMVCF constitutes tight junction strands in endothelial cells. Journal of Cell Biology. 1999;147:185-194.
- The molecular organization of endothelial junctions and their functional role in vascular morphogenesis and permeability. International Journal of Developmental Biology. 2000;44:743-748.
- Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92-96.
- Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nat Biotechnol. 2010;28:161-166.
- The Ki-67 protein: From the known and the unknown. Journal of Cellular Physiology. 2000;182:311-322.
- A Novel Assay for Apoptosis - Flow Cytometric Detection of Phosphatidylserine Expression on Early Apoptotic Cells Using Fluorescein-Labeled Annexin-V. Journal of Immunological Methods. 1995;184:39-51.
- Embryonic stem cell-derived embryoid bodies development in collagen gels recapitulates sprouting angiogenesis. Lab Invest. 2001;81:1669-1681.
- Cell adhesion and angiogenesis. Trends in Cell Biology. 1996;6:462-468.

