Nano to micro delivery systems: targeting angiogenesis in brain tumors
Journal of Angiogenesis Research. 2010;
Received: 9 July 2010 | Accepted: 8 October 2010 | Published: 8 October 2010
Vascular Cell ISSN: 2045-824X
Abstract
Treating brain tumors using inhibitors of angiogenesis is extensively researched and tested in clinical trials. Although anti-angiogenic treatment holds a great potential for treating primary and secondary brain tumors, no clinical treatment is currently approved for brain tumor patients. One of the main hurdles in treating brain tumors is the blood brain barrier - a protective barrier of the brain, which prevents drugs from entering the brain parenchyma. As most therapeutics are excluded from the brain there is an urgent need to develop delivery platforms which will bypass such hurdles and enable the delivery of anti-angiogenic drugs into the tumor bed. Such delivery systems should be able to control release the drug or a combination of drugs at a therapeutic level for the desired time. In this mini-review we will discuss the latest improvements in nano and micro drug delivery platforms that were designed to deliver inhibitors of angiogenesis to the brain.
Introduction
It is now evident that solid tumors beyond a given volume are dependent on the supply of oxygen and nutrients from the vascular system, which has to grow concomitantly with the tumor, similar to embryonic development. This process, of newly developed blood capillaries and blood vessels from pre-existing ones, has been termed angiogenesis and enables the tumor not only to increase its size but also its aggressiveness and its ability to metastasize [1–4]. The process of angiogenesis is implicated not only in the pathology of tumors but also in many other diseases including psoriasis [5, 6], age-related macular degeneration[7, 8] and rheumatoid arthritis [9].
Some of the most deadly malignancies that depend on the angiogenic process for their growth are primary brain tumors[10], among which glioblastoma multiforme (GBM) represents 40% of all cases. GBM has been targeted with many inhibitors of angiogenesis including tissue inhibitors of matrix metalloproteinases[11–13], chemokines [14–16], tyrosine kinase inhibitors [17–20], interleukins [21, 22], and naturally occurring proteolytic fragments of large precursor molecules such as endostatin, vasostatin, canstatin, angiostatin and others [23–29]. These molecules exert their inhibitory functions on endothelial cells by multiple mechanisms including proliferation, migration, protease activity, as well as the induction of apoptosis [30]. Although such angiogenesis inhibitors hold great promise, the ones that reached clinical trials for brain tumor patients have failed to achieve significant therapeutic outcome. One possible explanation for this outcome that is supported by many researchers is the lack of combinatory treatment with standard chemo and radiotherapy [31]. Another obstacle which may hamper the therapeutic outcome of anti-angiogenic therapy is the blood brain barrier (BBB, although destabilized in high grade GBM patients) which therapeutics need to bypass to exert a significant brain tumor inhibitory effect. The brain vasculature is predominantly different that of other tissues as its principal role is to prevent un-desirable and pathological substances from entering the brain parenchyma (Figure 1) [32]. The physical properties of the BBB, which include continuous tight junctions and low pinocytotic activity as well as high electrical resistance (attributed to occludin expression), form a tight barrier against materials with high molecular weight and ionic substances that can enter the brain parenchyma only through active transport [33, 34]. As such, the BBB hampers and complicates the systemic delivery of therapeutics to the brain [35, 36]. Small lipophilic drugs, which are expected to diffuse across the BBB, are removed from the central nerve system (CNS) by efflux transporters, such as P-glycoprotein (P-gp) [32, 37]. Other drug-based transporters that enable multi-drug resistance include the multi-drug resistance-associated protein (MRP) family (MRP1-MRP9) expressed in brain endothelial cells, breast cancer-resistant protein (ABCG2) [38, 39] and organic anion and cation transporters (OAT and OCT respectively) [40, 41].
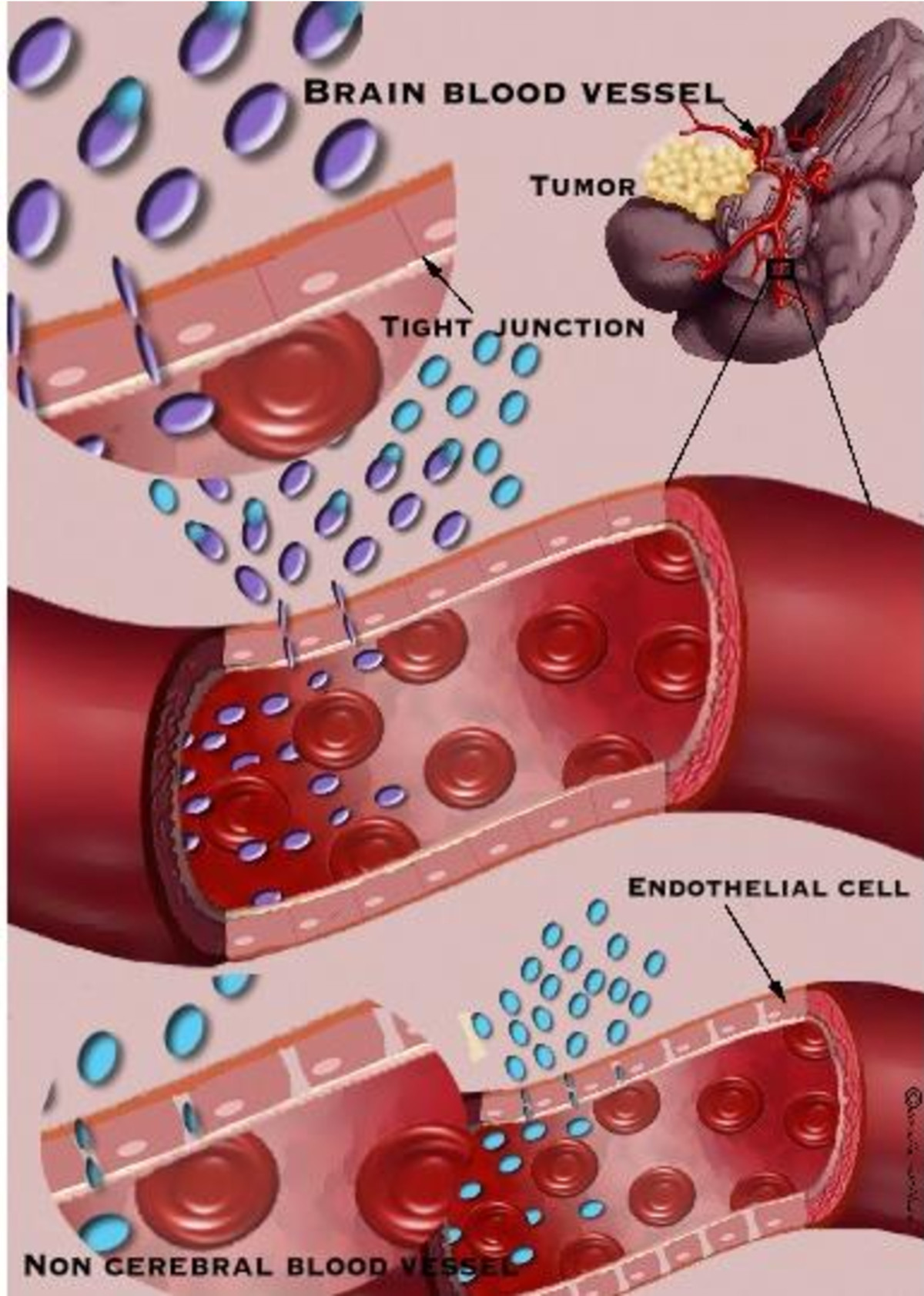
Figure 1
Figure 1 caption
Comparison between cerebral and noncerebral blood vessels. Cerebral blood vessel has tight junctions, which do not allow the passage of un-desirable and pathological substances to the brain parenchyma while non cerebral blood vessel allows better diffusion of drugs. Targeting the brain can be achieved using the drug itself or by using drug delivery platforms that release the drug at a specified location.
Nonetheless, it is also possible that the method of administration, which may destabilize the drugs, the therapeutic level needed to reach the tumor or a combination of all the above hurdles contribute to the disappointing outcome of such therapy approach.
These obstacles have resulted in a more urgent focus on developing alternative delivery modalities, which may bypass the BBB and efficiently target tumorangiogenesis while protecting and stabilizing the drug until reaching the tumor bed. These delivery modalities, may not only solve the problem of the BBB permeability and the need for a combinatorial treatment, but also reduce the therapeutic amount of drug needed to be delivered to the tumor, thus lowering toxicity and side effects of the drugs. Nevertheless such delivery modalities, whether local or systemic, have to deliver the therapeutics to the tumor mass, where the diffusion and distribution of the drug is governed by abnormal high tumor cell density, high interstitial fluid pressure within the tumors and leakiness of tumor microvasculature, resulting in fast clearance of the diffusing drug [42, 43].
In this review, we will discuss the progress made in designing and developing different nano and micro drug delivery platforms that aim to bypass the BBB and deliver systematically or locally therapeutics that target brain tumor angiogenesis. Table 1 summarizes the current clinical status of therapeutics, which are also utilized in studies aimed to develop drug delivery platforms for brain tumor therapy as will be discussed later on.
Table 1
FDA approved or under clinical trails drugs for brain tumors therapy
| Drug | FDA approved for brain tumors/Brain clinical trails | Approved routes of administration (Brain tumors & other disorders) | Drug delivery platforms in research for approved drugs |
|---|---|---|---|
| Temozolomide | Approved | Oral | Intracerebral Biodegradable gel matrices/Polymer nanoparticles |
| Procarbazine | Approved | Oral | - |
| lomustine | Approved | Oral | Liposomes/Microcapsules |
| Vincristine | Approved | IV | Intra-arterially |
| Carmustine | Approved | IV/Oral/Wafer | CED/Polymer microchips and microspheres |
| Carboplatin | Approved | IV | CED/Intracerebral/Intraarterial/Liposomes |
| Bevacizumab | Approved | IV | Intra-arterial |
| Doxorubicin | Phase I/II/III | injection;liposomal * | - |
| Imatinib mesylate | Phase I/II/III | Oral; Intravenous * | - |
| Cisplatin | Phase I/II/III | Injection * | - |
| Topotecan | Phase I/II/III | Injection; Oral * | - |
| Interferon-alpha | Phase I/II | Injection; Subcutaneous; Oral * | - |
| Paclitaxel | Phase I/II | Intravenous; Injection * | - |
| Arsenic trioxide | Phase I/II | Injection * | - |
Local drug delivery platforms
Local delivery to the brain utilizes BBB disruption, as well as local implantation of the delivery system directly in the tumor bed. These delivery systems which include cerebral infusion methods, polymeric nano-particles, wafers and more, are comprehensively studied and represent promising approaches for the delivery of drugs to the brain.
Intra-arterial cerebral infusion
Intra-arterial cerebral infusion involves the insertion of micro-catheters into the small arteries of the brain via the carotid artery [44]. This unique approach was used to infuse mannitol into the area of interest for transient disruption of the BBB, followed by the infusion of a therapeutic such as bevacizumab. As bevacizumab is selectively delivered to brain tumors, larger amount of the drug may be used when compared to the amount of drug used by intravenous administration of bevacizumab resulting with reduced side effects [44]. This delivery system may also enable the direct delivery of other drugs, which target the angiogenic processes in brain tumors.
Polymeric particles
Another popular approach, which is still in preclinical studies, is based on polymeric nano and micro-particles using polymers such as poly(butyl cyanoacrylate), Poly(ethylene-glycol), Poly(lactic-co-glycolic) acid, Poly-glycerol and others [45–48]. These particles can be loaded with or attached to different therapeutics and can then be delivered directly to the tumor site. The use of particle platforms significantly reduces (in some cases more than 50 folds) the amounts of drugs needed to reduce tumor volume and weight when compared to systemic administration. Such platforms need to be carefully designed, taking into account the properties of the drug carrier in term of immunogenicity, stability, preparation method, manufacturing costs, biodegradability and its pharmaceutical qualities (stability of the therapeutic, dosage capability, distribution and site specific targeting). These delivery vehicles must also retain the biological activity of the drug and allow its sustained release over extended periods of time when needed [49]. This is particularly important when attempting to deliver endogenous inhibitors of angiogenesis as opposed to chemotherapeutic drugs.
PLGA particles
One of the widely used polymers for the design of different delivery platforms is the poly-lactic-co-glycolic based polymer (PLGA). The huge advantages of delivery formulations based on PLGA, are their non immunogenicity and the ability to control the release profile of the drugs by manipulating the ratio of lactic to glycolic acids. An interesting publication by Shahani
In our lab, PLGA has been used to produce particles loaded with PEX, a fragment of matrix metalloproteinase (MMP)-2, or platelet factor 4 fragments (PF-4/CTF) for the delivery of these angiogenesis inhibitors to glioma bearing nude mice (Figure 2). PEX was detected and isolated from the culture medium of several cell lines and acts as inhibitor of angiogenesis, cell proliferation and migration, demonstrating a 99% suppression of glioma tumor growth in human glioma xenografts [13]. PF-4 is a strong anti-angiogenic factor that inhibits angiogenesis by blocking FGF-2 binding to endothelial cells [16, 53]. PEX and PF-4/CTF administered in this mode showed 88% and 95% reduction in tumor volume 30 days post treatment, respectively, demonstrating the advantage of PLGA microspheres for angiogenesis inhibitor delivery to glioma tumors [49].
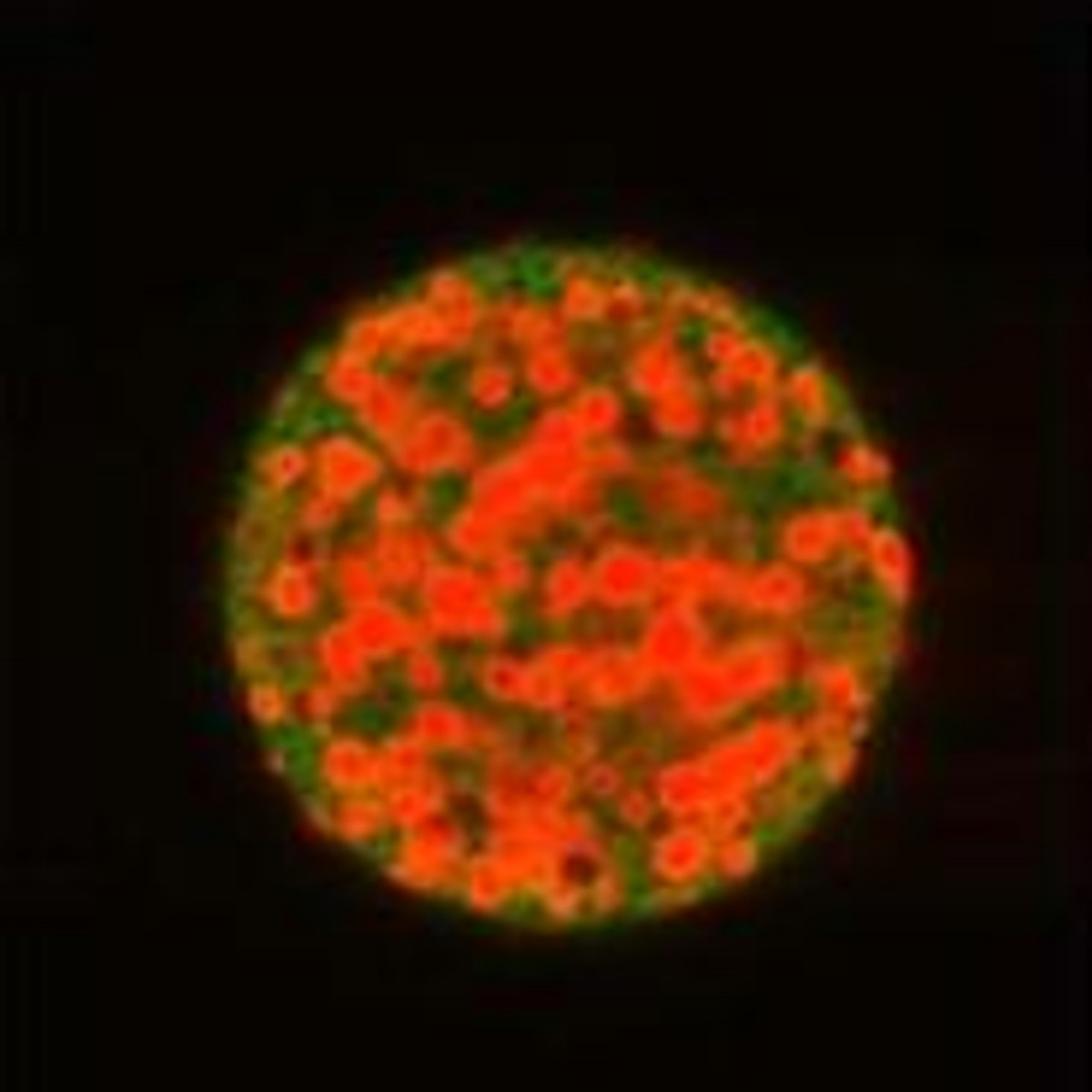
Figure 2
Figure 2 caption
PLGA particles loaded with therapeutic. PLGA particles (50:50 lactic to glycolic), labeled with 6-coumarin and loaded with rhodamine labeled PF-4/CTF.
Other drugs and proteins encapsulated in PLGA microspheres for the treatment of glioma include temozolomide [54], paclitaxel [55], imatinib mesylate for treating intracranial glioma xenografts [56], BCNU as an alternative for Gliadel [57], cisplatin [58], mitoxantrone [59] interleukin-18 [60] and 5-Fluorouracil [61, 62].
Wafers
Wafers are composed of biodegradable polymers that deliver the drugs into brain tumors via local administration, thus, bypassing the BBB. Such device may also be used for the delivery of angiogenesis inhibitors alone or in combination with other chemotherapeutic drugs.
The first device approved by the FDA was Gliadel®, a polymeric wafer designed for the delivery of carmustine [63]. Gliadel® is placed directly in the brain cavity created by the resection of the tumor. Clinical studies with Gliadel have shown increased survival rates in newly diagnosed malignant gliomas patient particularly when combined with a chemo-treatment [64, 65]. Due to the fact that not all of GBM patients are responsive to carmustine there is a need to evaluate other drugs for the treatment of GBM patients [66].
Cell delivery platform
Different studies have attempted to use cells, particularly stem cells, that are engineered to secret inhibitors of angiogenesis for brain tumor therapy [67, 68]. Another approach, which is based on cell delivery, is polymeric cell encapsulation. The encapsulation system consists of viable cells surrounded by a non-degradable, selectively permeable barrier that physically isolates the transplanted cells from host tissue and the immune system. This platform relies on host homeostatic mechanisms for the control of pH, metabolic waste removal, electrolytes and nutrients. One of the most studied cell micro-encapsulation methods has been based upon alginates, which are polysaccharides extracted from various species of brown algae (seaweed) and purified to a white powder. The alginates have different characteristics of viscosity and reactivity based on the specific algal source and the ions in the solution. Alginate has also hydrophilic properties, which minimize protein adsorption and cell adhesion, thus exhibit a high degree of biocompatibility. For cell encapsulation, the alginate gel is further complexed with polycations such as Poly-L-Lysine (PLL) to form a semi-permeable membrane, which allows the delivery of different bioactive substances to the surrounding while preventing the diffusion of antibodies and other components of the immune system. Cell encapsulation has been used for broad therapeutic applications such as delivery of neuroactive agents for the treatment of age-related degeneration [69, 70], Alzheimer's disease[71–74], amyotrophic lateral sclerosis [75, 76], neuroprotection [77], Huntington's disease [78, 79] and Parkinson's disease[80–82]. This approach has also been used to deliver inhibitors of angiogensis to glioma tumors. Read
Joki
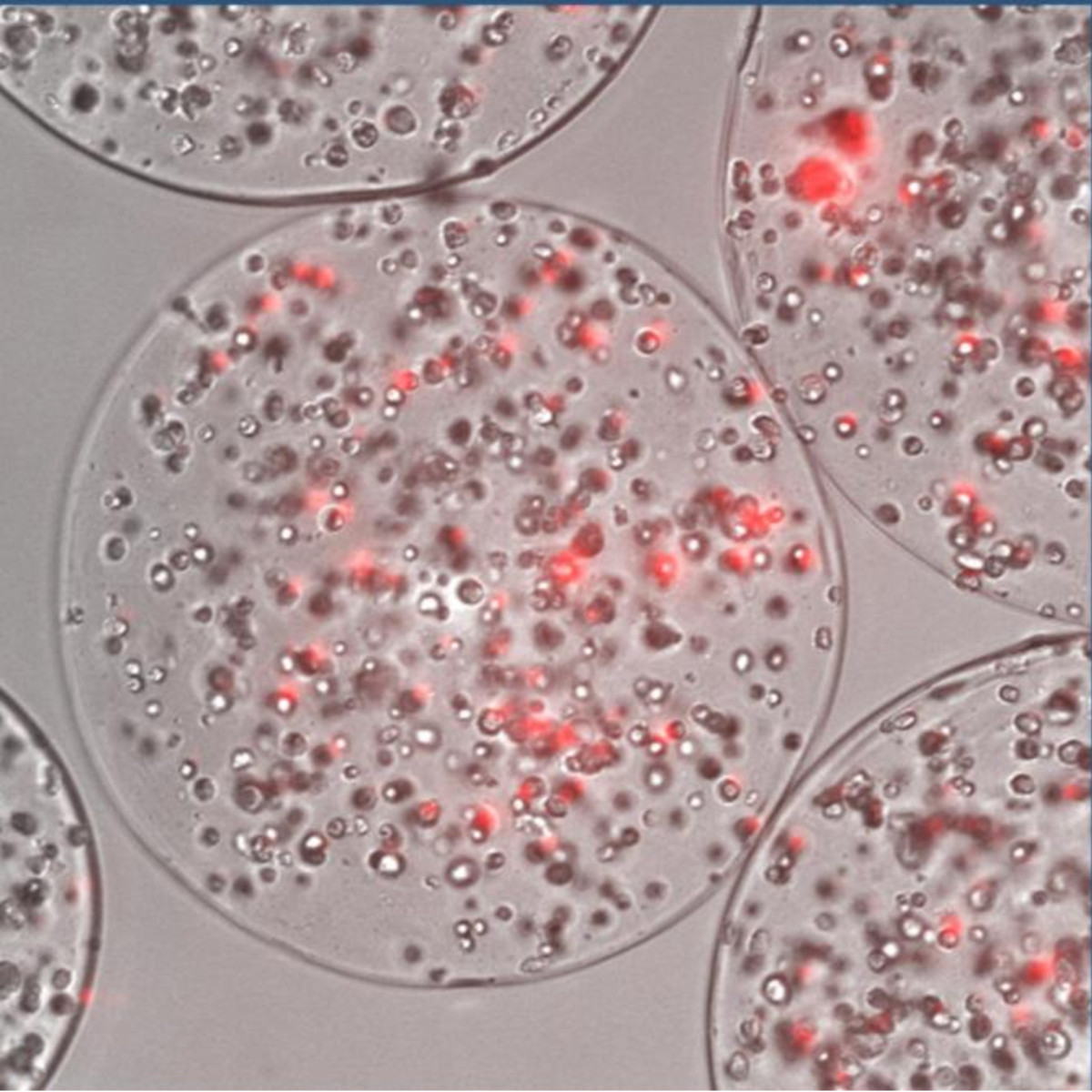
Figure 3
Figure 3 caption
Cell encapsulation platform. Human mesenchymal stem cells (hMSCs) labeled with the mCherry fluorescence marker engineered to express PEX and encapsulated in alginate capsules.
Convection-enhanced delivery
Convection-enhanced delivery (CED) was developed to overcome poor brain drug distribution. CED uses hydrostatic pressure to deliver drugs via a catheter located within or around a tumor. As poor drug diffusion through the brain interstitium restricts intratumoral drug administration, fluid convection within the brain (under pressure gradient), can greatly enhance the distribution of molecules in the tumor area. Distribution of drugs via CED is not restricted to white matter and penetration into gray matter can be observed 24 hours after infusion [90]. Nonetheless, Real-time monitoring of CED variability in efficacies as well as limited distribution of the drug still need to be addressed [91].
Saito
Systemic drug delivery platforms
Systemic drug delivery to the brain requires the consideration of the BBB as previously discussed. More over, systemic delivery systems need to overcome other obstacles such as protein adsorption, enzymatic digestion and engulfment of the delivery particles by phagocytic cells when using 300 nm-10 micrometer particle sizes. Nonetheless, systemic administration can have the benefit of non-invasiveness when compared to local administration using intracranial surgery. Different delivery systems have been developed to achieve systemic therapeutic targeting to the brain including pegylation of drugs, liposomes, polymeric nano-particles, dendrimers and bionanocapsules. We will discuss some of these designed nano-sized delivery systems, which may enable the delivery of drugs to the brain via systemic administration.
Drug Pegylation
One simplified approach designed to bypass the BBB is the modification of a drug by a polymeric composite, which some term as nano vehicles and other as drug conjugates. One example is the pegylation of interferon-alpha, which is known for its anti-angiogenic effects on tumors and other angiogenic diseases such as AIDS-related Kaposi sarcoma [46]. The motivation behind the pegylation of interferon-alpha was to both reduce its neurologic and immune system toxicities and to improve its circulation time [46, 48, 94, 95]. Pegylation of interferon alpha resulted in a long-lasting form of interferon that may target angiogenesis in glioma [94]. Pegylation has also been used on camptothecin and doxorubicin, improving their solubility, circulation time and lowering their toxicity [96, 97].
Liposomes
Liposomes are one of the most popular nano-system designs for systemic drug delivery. Liposomes are defined as delivery vehicles composed of phospholipids bilayers (one or more) ranging from tens to hundreds of nanometers in diameter (Figure 4). Liposomes' structure enables the entrapment of water soluble drugs at the aqueous core of the system, while hydrophobic drugs can be entrapped in the lipid bilayer composed of synthetic or natural lipids [98]. Liposomes have been widely researched for their ability to deliver proteins [99], chemotherapeutics [100, 101], RNA [102, 103], DNA [104] and other therapeutics. Their advantages include biocompatibility, low toxicity, enhanced efficacy of the encapsulated drugs and reduced side effects [105]. Fundamental disadvantage of conventional liposomes is their rapid removal from blood circulation by the mononuclear phagocyte system (MPS). Although this characteristic can be exploited to deliver drugs into phagocytic cells, it hampers the liposomal abilities to target the therapeutic to other cells and organs[106]. Liposomal clearance from the blood circulation is due to recognition of surface bounded opsonins by the MPS [107, 108] and membrane lysis of charged liposomes by complement components [109]. One approach to extend liposome circulation time and bypass such fast blood clearance, is to anchor Poly(ethylene glycol) (PEG), to the liposomal membrane- rendering them as stealth liposomes [110]. Adding PEG to the liposome preparations also decreases aggregation and reduces interactions with plasma proteins thus increases their circulation time [111, 112]. In gliomas, the BBB is disrupted at the site of the malignant lesion and the leaky endothelium enables passive convective transport of liposomes into the brain. Studies show that stealth liposomes extravasate into the extracellular space forming clusters and acting as a reservoir within the tumor area [113, 114]. Caelyx® is a novel formulation of stealth® liposomal doxorubicin. A study with 10 patients with metastatic brain tumors and five patients with brain glioblastoma undergoing radiotherapy confirmed intense accumulation of radio-labeled Caelyx® in the brain tumor as compared to the normal brain tissue[115]. In another study, doxorubicin encapsulated in liposomes exhibited break down of tumor vasculature [116]. Other drugs encapsulated in liposomes for treating brain tumors include taxol [117] and arsenic trioxide which down-regulated the expression of VEGF [118].
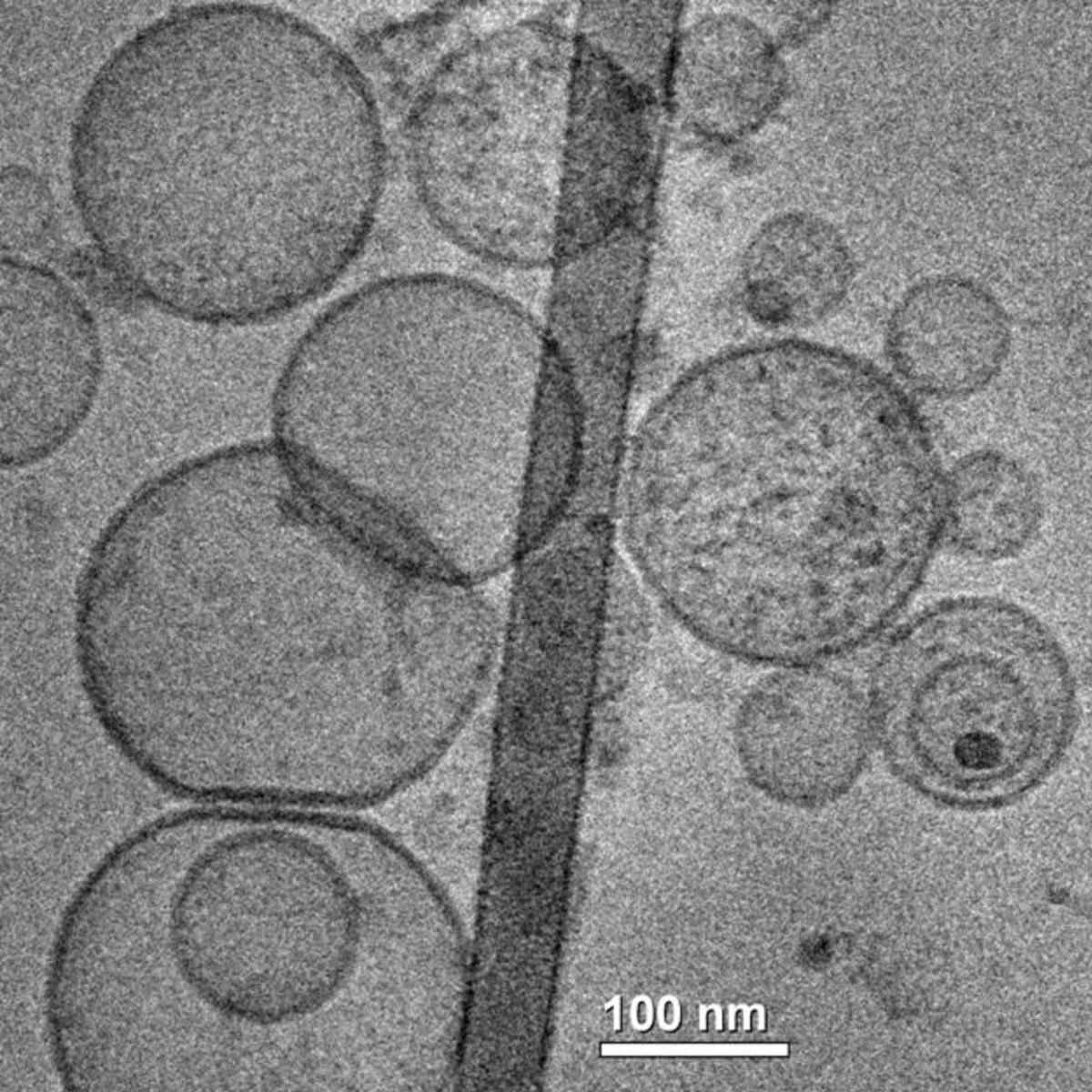
Figure 4
Figure 4 caption
Liposomes. TEM image of liposomes made of DOPE -1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, DMPA -1,2-dimyristoyl-sn-glycero-3-phosphate, POPE - 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine and POPC - 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine.
Polymeric nano-particles
Polymeric nano-particles (1-999 nano-meters) have attracted much attention as vehicles for systemic drug delivery due to their biocompatibility and stability properties. The diversity of materials, mostly synthetic, used to formulate nano-particles include poly(butyl cyanoacrylate), Poly(ethylene-glycol), Poly(lactic-co-glycolic) acid, Poly-glycerol and others [45–48]. Hekmatara
A different concept of nano-delivery system has been recently introduced and termed 'bionanocapsules' [125]. Bionanocapsulses are hollow nano-particles with an average diameter of 80 nm, displaying specific affinity to human hepatocytes via the pre-S1 peptide displayed on their surface and have successfully delivered peptides, genes and siRNA to the liver [125]. Tsutsui
Optimizing therapeutic delivery using bio-mathematical models
Mathematical models are based on the optimization of natural algorithms within the growing biomathematical tool kit and can provide a deeper understanding of the dynamic biological process involved with tumor angiogenesis, tumor growth or other tumor properties. Using mathematical modeling, tumor behavior upon drug treatment (free or released), from a delivery platform can be predicted. This is a powerful tool that can minimize invasive protocols, reduce drug quantities, force combinational drug treatments and reduce the number of animals required for
These methodologies have already been applied in the fields of cancer chemotherapy and cancer immunotherapy with minimization of tumor burden as their primary objective [127–130] and may be of great importance when considering GBM treatments. Gevertz
In our lab, Benny
Conclusions
Targeting brain tumor angiogenesis is a promising approach to arrest tumor growth. Nonetheless, clinical studies with inhibitors of angiogenesis have produced disappointing results. This may be due to the fact that these inhibitors were administered alone, without standard chemo and radiotherapy. It is also strongly possible that the way of administration (mostly intravenously), drug stability and quantities needed to achieve therapeutic outcome, hamper the therapeutic potential of such group of drugs. More over as the brain is a unique organ, protected via the BBB and efflux transporters (although disrupted in high grade glioma patients), the drugs need to bypass such barriers and reach the tumor in a therapeutic dosage. Therefore developing drug delivery platforms that bypass such barriers and target the therapeutics to the tumor site and on the other hand stabilize and protect the drug until it is released near or in the tumor bed can result in surprising therapeutic effects for such group of inhibitors.
The progress made in the field of biomaterials together with the pharmaceutical field, contribute vastly to the development of such local and systemic delivery platforms.
Yet, regardless of the enormous advantageous that these technologies can offer, the road to clinical studies using these platforms is still facing problems, which need to be studied and solved as they target the most protected and closed organ, the brain. Selection of polymers, preparation methods, drug loading and release profile, clearance of the drugs and immune aspects need to be taken in account and studied carefully to pave the way for new and promising delivery platform to reach the clinical setting.
Acknowledgements
We would like to acknowledge the Ed Sattel, Burt Richard and Marshall Mazer Research Funds for their support. We would like to acknowledge Danielle Machluf for contributing figure 1.
Figures 2, 3 and 4 are part of the PhD research theses of Ofra Benny, Amit Goren and Tomer Bronstin, respectively, conducted in our laboratory.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2
Authors’ original file for figure 3
Authors’ original file for figure 4
References
- Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186.
- Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007;11:539-554.
- Angiogenesis and gliomas: current issues and development of surrogate markers. Neurosurgery. 2008;62:31-50.
- Angiogenesis: an organizing principle for drug discovery?. Nat Rev Drug Discov. 2007;6:273-286.
- The role of angiogenesis in the pathogenesis of psoriasis. Autoimmunity. 2009;42:574-579.
- Angiogenesis drives psoriasis pathogenesis. Int J Exp Pathol. 2009;90:232-248.
- Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805-2816.
- Multiple effects of bevacizumab in angiogenesis: implications for its use in age-related macular degeneration. Acta Ophthalmol. 2009;87:517-523.
- Szekanecz Z, Besenyei T, Szentpetery A, Koch AE: Angiogenesis and vasculogenesis in rheumatoid arthritis. Curr Opin Rheumatol. 22: 299-306. 10.1097/BOR.0b013e328337c95a.
- Brain angiogenesis in developmental and pathological processes: mechanism and therapeutic intervention in brain tumors. Febs J. 2009;276:4653-4664.
- Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med. 1996;2:461-462.
- Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell. 1998;92:391-400.
- Simultaneous inhibition of glioma angiogenesis, cell proliferation, and invasion by a naturally occurring fragment of human metalloproteinase-2. Cancer Res. 2001;61:8730-8736.
- In vitro and in vivo systems to assess role of C-X-C chemokines in regulation of angiogenesis. Methods Enzymol. 1997;288:190-220.
- Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science. 1990;247:77-79.
- Inhibition of in vitro angiogenesis by platelet factor-4-derived peptides and mechanism of action. Blood. 1999;94:984-993.
- Complete inhibition of vascular endothelial growth factor (VEGF) activities with a bifunctional diabody directed against both VEGF kinase receptors, fms-like tyrosine kinase receptor and kinase insert domain-containing receptor. Cancer Res. 2001;61:7002-7008.
- Inhibition of both paracrine and autocrine VEGF/VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc Natl Acad Sci USA. 2001;98:10857-10862.
- Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841-844.
- Flk-1 as a target for tumor growth inhibition. Cancer Res. 1996;56:3540-3545.
- Interleukin 10 (IL-10) inhibition of primary human prostate cell-induced angiogenesis: IL-10 stimulation of tissue inhibitor of metalloproteinase-1 and inhibition of matrix metalloproteinase (MMP)-2/MMP-9 secretion. Clin Cancer Res. 1999;5:189-196.
- Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol. 1997;151:1105-1113.
- Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315-328.
- Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277-285.
- Endostatin inhibits VEGF-induced endothelial cell migration and tumor growth independently of zinc binding. Embo J. 1999;18:4414-4423.
- A gene therapy for cancer based on the angiogenesis inhibitor, vasostatin. Gene Ther. 2002;9:1207-1213.
- The angiogenesis inhibitor vasostatin does not impair wound healing at tumor-inhibiting doses. J Invest Dermatol. 2001;117:1036-1041.
- Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem. 2000;275:1209-1215.
- Angiostatin and endostatin: endogenous inhibitors of tumor growth. Cancer Metastasis Rev. 2000;19:181-190.
- Angiogenesis as a therapeutic target. Nature. 2005;438:967-974.
- Glioma angiogenesis: Towards novel RNA therapeutics. Cell Adh Migr. 2009;3:230-235.
- The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13:1663-1674.
- Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323-337.
- Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429:47-62.
- Drug targeting to the brain. Annu Rev Pharmacol Toxicol. 2007;47:323-355.
- Drug targeting. Breaking down barriers. Science. 2002;297:1116-1118.
- A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152-162.
- Plasma membrane localization of multidrug resistance-associated protein homologs in brain capillary endothelial cells. J Pharmacol Exp Ther. 2004;311:449-455.
- Characterisation of the brain multidrug resistance protein (BMDP/ABCG2/BCRP) expressed at the blood-brain barrier. Brain Res. 2003;971:221-231.
- Involvement of organic anion transporters in the efflux of uremic toxins across the blood-brain barrier. J Neurochem. 2006;96:1051-1059.
- Mechanisms of the penetration of blood-borne substances into the brain. Curr Neuropharmacol. 2009;7:142-149.
- Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583-592.
- Polymer implants for intratumoral drug delivery and cancer therapy. J Pharm Sci. 2008;97:1681-1702.
- Superselective intraarterial cerebral infusion of bevacizumab: a revival of interventional neuro-oncology for malignant glioma. J Exp Ther Oncol. 2009;8:145-150.
- Efficient systemic therapy of rat glioblastoma by nanoparticle-bound doxorubicin is due to antiangiogenic effects. Clin Neuropathol. 2009;28:153-164.
- Cytokine therapeutics: lessons from interferon alpha. Proc Natl Acad Sci USA. 1994;91:1198-1205.
- In vivo delivery of small interfering RNA to tumors and their vasculature by novel dendritic nanocarriers. Faseb J. 2010;9:3122-3134.
- Brain tumor therapy by combined vaccination and antisense oligonucleotide delivery with nanoparticles. J Neuroimmunol. 2008;195:21-27.
- Continuous delivery of endogenous inhibitors from poly(lactic-co-glycolic acid) polymeric microspheres inhibits glioma tumor growth. Clin Cancer Res. 2005;11:768-776.
- Shahani K, Swaminathan SK, Freeman D, Blum A, Ma L, Panyam J: Injectable sustained release microparticles of curcumin: a new concept for cancer chemoprevention. Cancer Res. 70: 4443-4452. 10.1158/0008-5472.CAN-09-4362.
- Arai T, Benny O, Joki T, Menon LG, Machluf M, Abe T, Carroll RS, Black PM: Novel local drug delivery system using thermoreversible gel in combination with polymeric microspheres or liposomes. Anticancer Res. 30: 1057-1064.
- Development of new polymer-based particulate systems for anti-glioma vaccination. Int J Pharm. 2006;309:1-5.
- A short peptide domain of platelet factor 4 blocks angiogenic key events induced by FGF-2. Faseb J. 2001;15:550-552.
- Temozolomide/PLGA microparticles: a new protocol for treatment of glioma in rats. >Med Oncol. 2010.
- Hydrogel matrix entrapping PLGA-paclitaxel microspheres: drug delivery with near zero-order release and implantability advantages for malignant brain tumour chemotherapy. Pharm Res. 2009;26:2101-2114.
- Local delivery of poly lactic-co-glycolic acid microspheres containing imatinib mesylate inhibits intracranial xenograft glioma growth. Clin Cancer Res. 2009;15:1222-1231.
- Three weeks release BCNU loaded hydrophilic-PLGA microspheres for interstitial chemotherapy: Development and activity against human glioblastoma cells. J Microencapsul. 2008;25:561-568.
- Biodegradable microparticles and fiber fabrics for sustained delivery of cisplatin to treat C6 glioma in vitro. J Biomed Mater Res A. 2008;85:897-908.
- Treatment of malignant gliomas with mitoxantrone-loaded poly (lactide-coglycolide) microspheres. Neurosurgery. 2006;59:1296-1302.
- Development and characterization of interleukin-18-loaded biodegradable microspheres. Int J Pharm. 2006;314:179-188.
- Effect of stereotactic implantation of biodegradable 5-fluorouracil-loaded microspheres in healthy and C6 glioma-bearing rats. Neurosurgery. 1996;39:117-123.
- Local and sustained delivery of 5-fluorouracil from biodegradable microspheres for the radiosensitization of malignant glioma: a randomized phase II trial. Neurosurgery. 2005;56:242-248.
- Recent advances in brain tumor therapy: local intracerebral drug delivery by polymers. Invest New Drugs. 2004;22:27-37.
- Balossier A, Dorner L, Emery E, Heese O, Mehdorn HM, Menei P, Singh J: Incorporating BCNU wafers into malignant glioma treatment: European case studies. Clin Drug Investig. 30: 195-204.
- Menei P, Metellus P, Parot-Schinkel E, Loiseau H, Capelle L, Jacquet G, Guyotat J: Biodegradable carmustine wafers (Gliadel) alone or in combination with chemoradiotherapy: the French experience. Ann Surg Oncol. 17: 1740-1746. 10.1245/s10434-010-1081-5.
- Biodegradable polymer implants to treat brain tumors. J Control Release. 2001;74:63-67.
- Antiangiogenic therapy against experimental glioblastoma using genetically engineered cells producing interferon-alpha, angiostatin, or endostatin. Hum Gene Ther. 2003;14:883-895.
- The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62:5657-5663.
- Intrastriatal implants of polymer-encapsulated PC12 cells: effects on motor function in aged rats. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:935-946.
- Alleviation of behavioral deficits in aged rodents following implantation of encapsulated GDNF-producing fibroblasts. Brain Res. 1996;736:99-110.
- Transplantation of a polymer-encapsulated cell line genetically engineered to release NGF. Exp Neurol. 1993;122:100-106.
- Polymer-encapsulated cells genetically modified to secrete human nerve growth factor promote the survival of axotomized septal cholinergic neurons. Proc Natl Acad Sci USA. 1994;91:2324-2328.
- Protective effect of encapsulated cells producing neurotrophic factor CNTF in a monkey model of Huntington's disease. Nature. 1997;386:395-399.
- Garcia P, Youssef I, Utvik JK, Florent-Bechard S, Barthelemy V, Malaplate-Armand C, Kriem B, Stenger C, Koziel V, Olivier JL, et al: Ciliary neurotrophic factor cell-based delivery prevents synaptic impairment and improves memory in mouse models of Alzheimer's disease. J Neurosci. 30: 7516-7527. 10.1523/JNEUROSCI.4182-09.2010.
- Intrathecal delivery of CNTF using encapsulated genetically modified xenogeneic cells in amyotrophic lateral sclerosis patients. Nat Med. 1996;2:696-699.
- Evaluation of an intrathecal immune response in amyotrophic lateral sclerosis patients implanted with encapsulated genetically engineered xenogeneic cells. Cell Transplant. 2000;9:471-484.
- Central nervous system delivery of recombinant ciliary neurotrophic factor by polymer encapsulated differentiated C2C12 myoblasts. Hum Gene Ther. 1996;7:2135-2146.
- Delivery of neurotrophic factors to the CNS using encapsulated cells: developing treatments for neurodegenerative diseases. Cell Transplant. 1995;4(Suppl 1):S27-28.
- Transplants of encapsulated rat choroid plexus cells exert neuroprotection in a rodent model of Huntington's disease. Cell Transplant. 2008;16:987-992.
- Cellular delivery of human CNTF prevents motor and cognitive dysfunction in a rodent model of Huntington's disease. Cell Transplant. 1997;6:249-266.
- Somatic delivery of catecholamines in the striatum attenuate parkinsonian symptoms and widen the therapeutic window of oral sinemet in rats. Exp Neurol. 1997;145:130-140.
- Encapsulated cell biodelivery of GDNF: a novel clinical strategy for neuroprotection and neuroregeneration in Parkinson's disease?. Exp Neurol. 2008;209:82-88.
- Local endostatin treatment of gliomas administered by microencapsulated producer cells. Nat Biotechnol. 2001;19:29-34.
- Intravital microscopy reveals novel antivascular and antitumor effects of endostatin delivered locally by alginate-encapsulated cells. Cancer Res. 2001;61:6830-6837.
- Cell therapy using encapsulated cells producing endostatin. Acta Neurochir Suppl. 2003;88:137-141.
- Continuous release of endostatin from microencapsulated engineered cells for tumor therapy. Nat Biotechnol. 2001;19:35-39.
- Goren A, Dahan N, Goren E, Baruch L, Machluf M: Encapsulated human mesenchymal stem cells: a unique hypoimmunogenic platform for long-term cellular therapy. Faseb J. 24: 22-31. 10.1096/fj.09-131888.
- The efficacy of alginate encapsulated CHO-K1 single chain-TRAIL producer cells in the treatment of brain tumors. J Neurooncol. 2006;78:31-39.
- Cell encapsulation technology as a therapeutic strategy for CNS malignancies. Neuro Oncol. 2001;3:201-210.
- Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91:2076-2080.
- New strategies to deliver anticancer drugs to brain tumors. Expert Opin Drug Deliv. 2009;6:1017-1032.
- Convection-enhanced delivery of Ls-TPT enables an effective, continuous, low-dose chemotherapy against malignant glioma xenograft model. Neuro Oncol. 2006;8:205-214.
- Combinatorial antiangiogenic gene therapy by nonviral gene transfer using the sleeping beauty transposon causes tumor regression and improves survival in mice bearing intracranial human glioblastoma. Mol Ther. 2005;12:778-788.
- Cognitive effects of pegylated interferon in individuals with primary brain tumors. J Neurooncol. 2009;95:231-237.
- Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568-572.
- Camptothecin delivery systems: the antitumor activity of a camptothecin-20-0-polyethylene glycol ester transport form. Anticancer Res. 1997;17:3361-3368.
- Zhu S, Hong M, Zhang L, Tang G, Jiang Y, Pei Y: PEGylated PAMAM dendrimer-doxorubicin conjugates: in vitro evaluation and in vivo tumor accumulation. Pharm Res. 27: 161-174. 10.1007/s11095-009-9992-1.
- Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1:297-315.
- Preparation, characterization, and in vivo evaluation of nanoliposomes-encapsulated bevacizumab (avastin) for intravitreal administration. Retina. 2009;29:699-703.
- Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002;4:95-99.
- Co-delivery of doxorubicin and plasmid by a novel FGFR-mediated cationic liposome. Int J Pharm. 2010;393(1-2):119-26.
- Barichello JM, Ishida T, Kiwada H: Complexation of siRNA and pDNA with cationic liposomes: the important aspects in lipoplex preparation. Methods Mol Biol. 605: 461-472. full_text.
- Convenient targeting of stealth siRNA-lipoplexes to cells with chelator lipid-anchored molecules. J Control Release. 2009;139:229-238.
- Blood-brain barrier delivery of protein and non-viral gene therapeutics with molecular Trojan horses. J Control Release. 2007;122:345-348.
- Long-circulating liposomes. Crit Rev Ther Drug Carrier Syst. 1994;11:231-270.
- Macrophage specific drug delivery in experimental leishmaniasis. Curr Mol Med. 2004;4:681-689.
- Serum opsonins and liposomes: their interaction and opsonophagocytosis. Crit Rev Ther Drug Carrier Syst. 1992;9:39-90.
- Beta 2 glycoprotein I is a major protein associated with very rapidly cleared liposomes in vivo, suggesting a significant role in the immune clearance of "non-self" particles. J Biol Chem. 1995;270:25845-25849.
- Liposome-complement interactions in rat serum: implications for liposome survival studies. Biochim Biophys Acta. 1994;1191:43-51.
- Controlling the physical behavior and biological performance of liposome formulations through use of surface grafted poly(ethylene glycol). Biosci Rep. 2002;22:225-250.
- Repulsive interactions and mechanical stability of polymer-grafted lipid membranes. Biochim Biophys Acta. 1992;1108:40-48.
- Molecular mechanism of the lipid vesicle longevity in vivo. Biochim Biophys Acta. 1993;1146:157-168.
- Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54:3352-3356.
- Doxorubicin encapsulated in sterically stabilized liposomes for the treatment of a brain tumor model: biodistribution and therapeutic efficacy. J Neurosurg. 1995;83:1029-1037.
- High intratumoural accumulation of stealth liposomal doxorubicin (Caelyx) in glioblastomas and in metastatic brain tumours. Br J Cancer. 2000;83:1281-1286.
- Antivasculature effects of doxorubicin-containing liposomes in an intracranial rat brain tumor model. Cancer Res. 2002;62:2561-2566.
- Effects of free and liposome-encapsulated taxol on two brain tumors xenografted into nude mice. In Vivo. 1992;6:23-27.
- Intravenous administration of arsenic trioxide encapsulated in liposomes inhibits the growth of C6 gliomas in rat brains. J Chemother. 2008;20:253-262.
- Downregulation of uPA, uPAR and MMP-9 using small, interfering, hairpin RNA (siRNA) inhibits glioma cell invasion, angiogenesis and tumor growth. Neuron Glia Biol. 2004;1:165-176.
- Intraperitoneal injection of a hairpin RNA-expressing plasmid targeting urokinase-type plasminogen activator (uPA) receptor and uPA retards angiogenesis and inhibits intracranial tumor growth in nude mice. Clin Cancer Res. 2007;13:4051-4060.
- Adenovirus-mediated transfer of siRNA against MMP-2 mRNA results in impaired invasion and tumor-induced angiogenesis, induces apoptosis in vitro and inhibits tumor growth in vivo in glioblastoma. Oncogene. 2008;27:4830-4840.
- Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769-773.
- miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell. 2008;14:382-393.
- Direct evidence that polysorbate-80-coated poly(butylcyanoacrylate) nanoparticles deliver drugs to the CNS via specific mechanisms requiring prior binding of drug to the nanoparticles. Pharm Res. 2003;20:409-416.
- Nanoparticles for the delivery of genes and drugs to human hepatocytes. Nat Biotechnol. 2003;21:885-890.
- Development of bionanocapsules targeting brain tumors. J Control Release. 2007;122:159-164.
- Development of optimal drug administration strategies for cancer-chemotherapy in the framework of systems theory. Int J Biomed Comput. 1984;15:139-150.
- Therapy burden, drug resistance, and optimal treatment regimen for cancer chemotherapy. IMA J Math Appl Med Biol. 2000;17:33-51.
- Cancer immunotherapy by interleukin-21: potential treatment strategies evaluated in a mathematical model. Cancer Res. 2006;66:7293-7300.
- Optimization of interleukin-21 immunotherapeutic strategies. J Theor Biol. 2007;248:259-266.
- Modeling the effects of vasculature evolution on early brain tumor growth. J Theor Biol. 2006;243:517-531.
- Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27-31.
- Improving alloreactive CTL immunotherapy for malignant gliomas using a simulation model of their interactive dynamics. Cancer Immunol Immunother. 2008;57:425-439.

