Angiogenic potential of endothelial progenitor cells and embryonic stem cells
Vascular Cell. 2011;
Received: 9 March 2011 | Accepted: 11 May 2011 | Published: 11 May 2011
Vascular Cell ISSN: 2045-824X
Abstract
Background
Endothelial progenitor cells (EPCs) are implicated in a range of pathological conditions, suggesting a natural therapeutic role for EPCs in angiogenesis. However, current angiogenic therapies involving EPC transplantation are inefficient due to rejection of donor EPCs. One solution is to derive an expanded population of EPCs from stem cells
Results
The effect on tubule complexity and longevity varied with transplantation quantity: significant effects were observed when tubules were transplanted with a quantity of EPCs equivalent to 50% of the number of ECs originally seeded on to the assay gel but not with 10% EPC transplantation. Gene expression of the endothelial markers VEGFR2, VE-cadherin and CD31, determined by qPCR, also changed dynamically during transplantation. ECCM-treated ESC-derived progenitor cells exhibited angiogenic potential, demonstrated by
Conclusions
We concluded the effect of EPCs is cumulative and beneficial, relying on upregulation of the angiogenic activity of transplanted cells combined with an increase in proliferative cell number to produce significant effects upon transplantation. Furthermore, EPCs derived from ESCs may be developed for use as a rapidly-expandable alternative for angiogenic transplantation therapy.
Background
In the early embryo, mesodermal stem cells in the bone marrow (BM) differentiate to form haemangioblasts, the common precursor of haematopoietic stem cells and endothelial-lineage angioblasts [1, 2]. During vasculogenesis these immature but lineage-committed angioblasts, termed endothelial progenitor cells (EPCs), migrate and congregate into clusters, called blood islands, forming the primary vascular plexus from which a complex microcirculation arises [3, 4]. In contrast, adult vascular growth occurs primarily through angiogenesis whereby new capillaries develop endogenously from fully-differentiated endothelial cells (ECs) within existing vessels [5]. However, angiogenesis is not the sole mechanism by which the adult vasculature is augmented [6, 7]. Circulating EPCs share phenotypic characteristics with embryonic EPCs [8] and incorporate into sites of neovascularisation, suggesting a role for EPCs in angiogenic renewal [9, 10]. They express endothelial-specific markers, including vascular endothelial growth factor receptor 2 (VEGFR2), CD31, CD133, VE-cadherin and von Willebrand factor (vWF), which have various roles in cell-cell adhesion, vascular permeability and the modulation of other cellular responses during angiogenesis [11, 12]. Indeed, EPCs are implicated in angiogenesis stimulated by conditions such as coronary artery disease and myocardial infarction, confirmed by clinical observations of EPC mobilisation in such patients and incorporation into foci of pathological neovascularisation [13, 14].
Nevertheless, current approaches to angiogenic therapies are problematic. Endogenous approaches most likely rely on the recruitment of circulating EPCs, and the delivery of a single pro-angiogenic substance is insufficient to elicit the complete and prolonged response necessary for effective angiogenesis [15, 16]. Exogenous therapies involve administration of allogeneic donor EPCs, which with poor HLA matching leads to increased immune rejection resulting in reduced transplantation efficiency [17, 18]. Consequently, the use of an expanded population of autologous EPCs from the patient's own adult stem cell population is desirable.
In contrast to EPCs, the inner cell mass of blastocyst-stage embryos gives rise to a population of self renewing pluripotent, embryonic stem cells (ESCs) [19], which are precursors to all cell types of the body [20]. Whilst potential ethical considerations must be considered, such as the use of otherwise viable human embryos for cell harvesting, the capacity of ESCs for unlimited growth may allow rapid
Chemical stimuli may, at least in part, drive the response of EPCs during angiogenesis. These stimuli can be released from surrounding tissues, i.e. from within the microenvironment, or from the endothelial cells themselves. Indeed, it has been shown in hypoxic wounds of diabetic patients that EPCs in the BM respond by following chemokine gradients, resulting in their homing to sites of hypoxia where they can participate in neovascularisation [24]. Consequently, the microenvironment in which progenitor cells are cultured is critical to their ability to maintain their progenitor status, i.e. to self-renew and give rise to differentiated cell types as and when recruited to do so. For instance, it has been shown that under the appropriate microenvironmental cues, achieved by using a combination of
Although the remodelling of mature ECs during the development of endothelial tubules
Methods
Cell lines and culture media
The murine EC line MCEC-1 was provided by Prof. Gerard Nash (University of Birmingham, UK). The murine EPC line MFLM-4 was obtained from Seven Hills Bioreagents (Ohio, US). Endothelial growth medium consisted of Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin and 2 mM L-glutamine, supplemented with 10 U·ml-1 heparin and 0.1 μg·ml-1 recombinant murine epidermal growth factor (EGF) (for MCEC-1) or 25 mg·ml-1 amphotericin B and 10 ng·ml-1 basic fibroblast growth factor (bFGF) (for MFLM-4). ESCs derived from blastocysts of 129S2/SvPas mice (D3) were obtained from American Type Culture Collection (ATCC, Middlesex, UK). ESC growth medium consisted of Knockout DMEM (Invitrogen, Paisley, UK) containing 15% ESC-Screened FBS (HyClone; Thermo Fisher, Leicestershire, UK), 1% penicillin-streptomycin, 2 mM L-glutamine, 1% non-essential amino acids, 0.1 mM β-mercaptoethanol and 10 ng·μl-1 recombinant murine leukemia inhibitory factor (LIF) (Chemicon, Livingston, UK). Undifferentiated ESCs were maintained on CF-1 murine embryonic fibroblast feeder cells (ATCC) mitotically-inactivated by treatment with 10 μg·ml-1 mitomycin C for 2 h at 37°C.
Differentiation of stem cells
ESCs were spontaneously differentiated using the hanging droplet method [29]. Undifferentiated cells (D0) were resuspended in growth medium (minus LIF) and plated as 20 μl droplets (450 cells/drop) on Petri dishes. Inverted dishes were incubated at 37°C/5% CO2 for 48 hours (D1-2) to induce embryoid body (EB) formation. EBs were then grown in suspension for 5 days (D3-7). For directed differentiation, cells were resuspended in ECCM for D3-7; ECCM was produced by 24 h incubation with MCEC-1 cells.
In vitrotubule formation assay
Angiogenic activity of cells was assessed using the
In vitroEPC transplantation
Transplantation was performed by addition of EPCs into a tubule formation assay containing ECs. Prior to transplantation, EPCs were labelled using 5 mM Qtracker 655 quantum dot (Qdot) labelling solution (Invitrogen, Paisley, UK) according to manufacturer's instructions. To investigate the effect of cell number on transplantation two quantities of EPCs were used, equivalent to 10% (8 × 103 cells) or 50% (4 × 104 cells) of the number of ECs originally seeded. At 5 h, a suspension of 10% or 50% Qdot-labelled EPCs in 50 μl PBS was pipetted evenly over the EC-containing ECMatrix gel and the coverslip was returned to the incubator.
Quantification of in vitrotubule formation
Tubule formation was quantified by counting nodes, points at which branches intersect, and measuring branch lengths. Nodes were graded based on structure: branch end-points as N1; intersections as N2; junctions of three or four branches as N3 or N4; and nodes of five or more branches as N5+. The average number of each node type was calculated based on five fields of view every 2 h. Branch length was measured using AQuaL Angiogenesis Quantification software [30]. Tubule images were tagged with markers, defined as end-points or junctions, and overall lengths automatically calculated based on five fields of view.
Gene expression analysis
Cells were recovered from ECMatrix gel using Cell Recovery Solution (BD Biosciences, Oxford, UK). Total RNA was isolated using RNAqueous-4PCR Kit (Ambion, Warrington, UK) followed by decontamination with DNase I (Ambion). Reverse transcription was performed using MMLV reverse transcriptase (Bioline, London, UK) with Oligo (dT)18 primers to produce complementary DNA (cDNA). cDNA was used for quantitative real-time PCR (qPCR) performed in a Corbett Rotor-Gene 3000 cycler (QIAGEN, West Sussex, UK) using SYBR Green chemistry. Each qPCR reaction (25 μl final volume) consisted of 1 μl cDNA template, 12.5 μl Quantace SensiMix (Bioline, London, UK), 0.5 μl 50× SYBR Green I Solution (Bioline), 0.2 μM each of forward and reverse primers (Invitrogen) and PCR-grade H2O. A 270 bp product of
Immunocytochemistry (ICC)
Cells were fixed using 4% paraformaldehyde and non-specific blocking performed using 2% bovine serum albumin, 100 mM glycine and 0.2 mg·ml-1 sodium azide for 30 minutes at room temperature. Primary antibodies included VEGFR2 (goat, 4 μg·ml-1), CD133 (rabbit, 2 μg·ml-1) and CD34 (rat, 5 μg·ml-1) (Abcam, Cambridge, UK). Secondary antibodies included anti-goat conjugated to Alexa Fluor (AF) 488 (rabbit; 1:400), anti-rabbit (AF 594, donkey; 1:500) and anti-rat (AF 594, chicken; 1:400) (Molecular Probes; Invitrogen). Coverslips were mounted using Vectorshield DAPI Mounting Medium (Vector Laboratories Inc., Peterborough, UK). Microscopy was performed using a Zeiss 510 Meta confocal microscope (Carl Zeiss Ltd., Hertfordshire, UK) with ×63 oil DIC objective (NA 1.4) with the detection pinhole adjusted to 1 Airy unit. DAPI was excited at 360 nm and detected at 460 nm, Alexa Fluor (AF) 488 was excited at 488 nm and detected between 515-545 nm, and AF 594 was excited at 594 nm and detected at 617 nm. Qdot-labelled EPCs were visualized by excitation between 405-615 nm and detection at 655 nm.
Statistical methods
Statistical differences were calculated using one-way ANOVA and post hoc multiple comparisons using the Bonferroni test.
Results
Endothelial-specific gene expression is dynamic during lineage development
Highly-confluent cells, including the endothelium, demonstrate a reduced response to specific growth factors and proliferative signals such as VEGF [31, 32] and contact inhibition is thus implicated as an important endothelial anti-proliferative mechanism. Hence, to determine the effect of contact inhibition on endothelial-specific expression, we analysed the patterns of expression of three key endothelial markers,
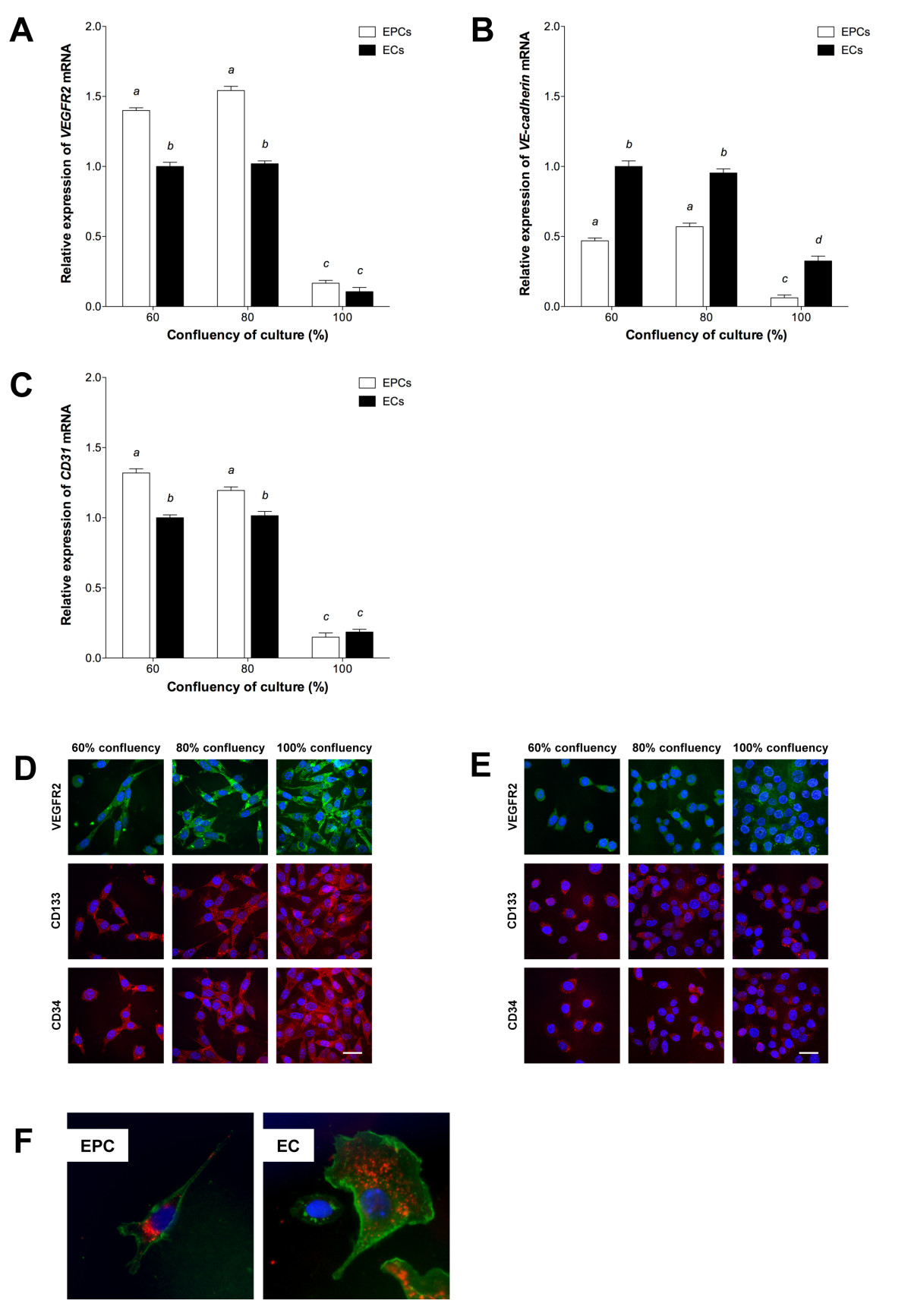
Figure 1
Figure 1 caption
Characterization of EPCs and ECs. qPCR of (A) , (B) and (C) expression at increasing confluency, relative to 60% confluent ECs (mean ± SEM; n = 3). Columns with different letters are significantly different (P < 0.05). ICC of VEGFR2, CD133 and CD34 in (D) EPCs and (E) ECs. Scale bar = 30 μm. (F) Uptake of DiI-ac-LDL (red) and staining with lectin (green). Nuclear staining (blue) with DAPI-containing mounting medium.
Further to the characterisation of the chosen cell lines by gene expression analysis, the endothelial phenotypes of MFLM-4 EPCs and MCEC-1 ECs was demonstrated by endothelial-specific lectin staining and uptake of DiI-labelled ac-LDL (Figure 1f).
EPCs and ECs demonstrate angiogenic potential by in vitrotubule formation
To determine whether EPCs and ECs possessed angiogenic potential, and were thus able to form endothelial tubules, we cultured both cell types individually on ECMatrix gel for 14 h. Tubule formation was quantified by node counting and branch length measurement. The mean number of each node type at each time-point throughout the assay was similar for EPCs and ECs (P > 0.05; Figure 2a and 2b, respectively). A large number of N1 nodes was observed at 2 h, decreasing as the assay progressed, resulting in the formation of more N2 and N3 nodes and thus more complex structures. These were maximal at 4 h and 6 h, respectively for both cell lines. After 6 h, the number of N2 and N3 nodes decreased and they were last evident between 10 h and 12 h. Nevertheless, this gave rise to the formation of more complex structures, N4 nodes, which were first observed at 4 h for both EPCs and ECs and reached a maximum at 6 h and persisted until 8 h (ECs) and 10 h (EPCs) (P < 0.05). In turn, the most complex nodal formation (N5+) was first evident in EPC colonies between 6 to 8 h whilst ECs did not produce these structures. However, no tubule networks were observed after 12 h for either EPCs or ECs. The pattern of tubule formation indicated by branch length measurement was also similar for EPCs and ECs (Figure 2c). Mean length increased for both cell types from 0 to 6 h with maximal branch length being at 6 h, although mean length of EPC tubules at 6 h was significantly greater (P < 0.01). Nevertheless, mean branch length then decreased with significantly lower length being observed for ECs at 8 h (P < 0.05) when compared to EPCs. Thereafter, mean branch length continued to decrease for both cell types.
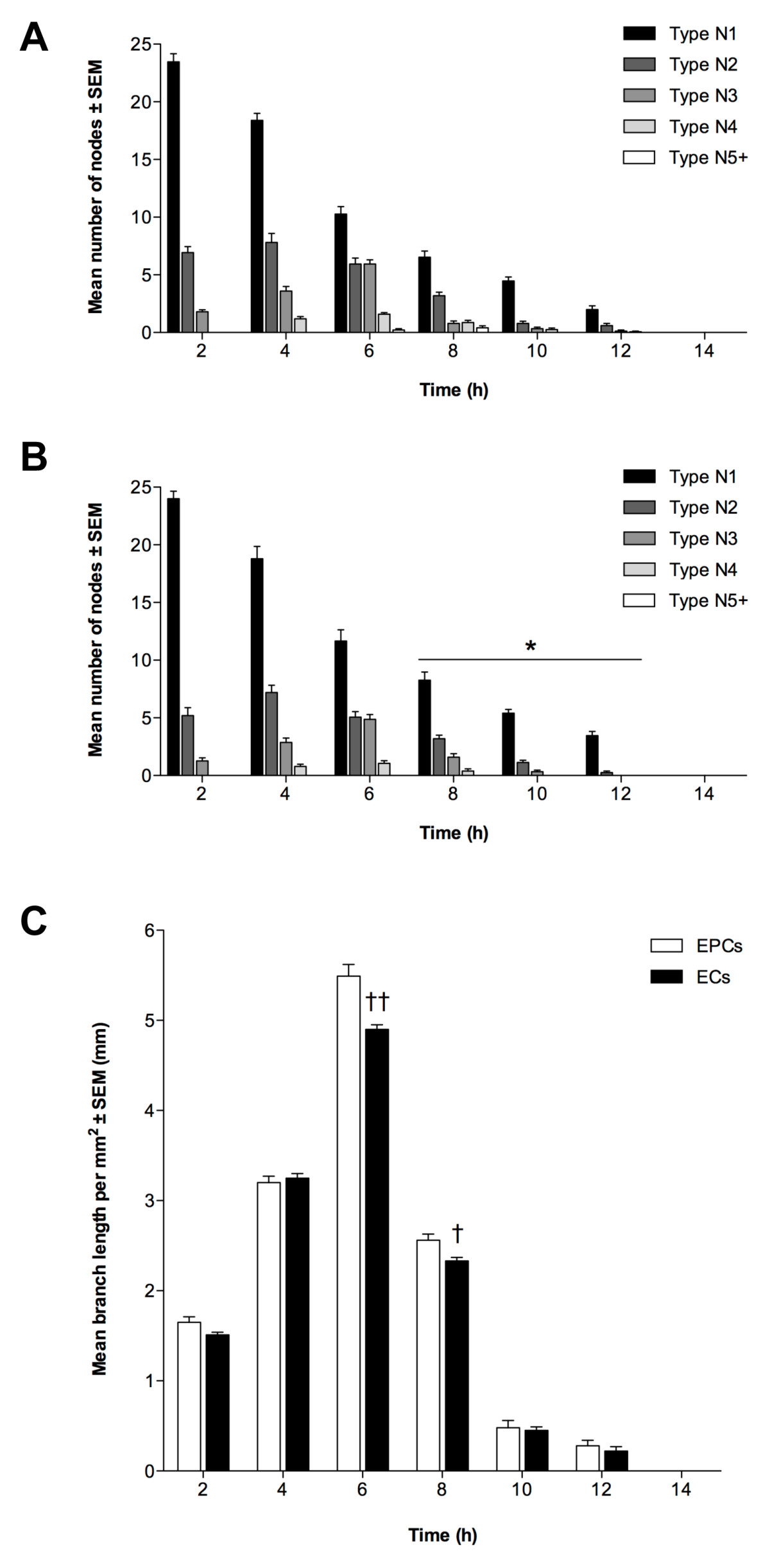
Figure 2
Figure 2 caption
Angiogenic potential of EPCs and ECs. Tubule formation of (A) EPCs and (B) ECs quantified by node counting. Presented as mean node number ± SEM (n = 3); *P < 0.05 vs. EPC nodes at same time-point. (C): Branch measurements, as mean length per mm ± SEM (n = 3); †P < 0.05, ††P < 0.01 vs. EPC branch length at same time-point.
To determine whether tubule formation from EPCs and ECs was a function of endothelial-specific expression, we analyzed
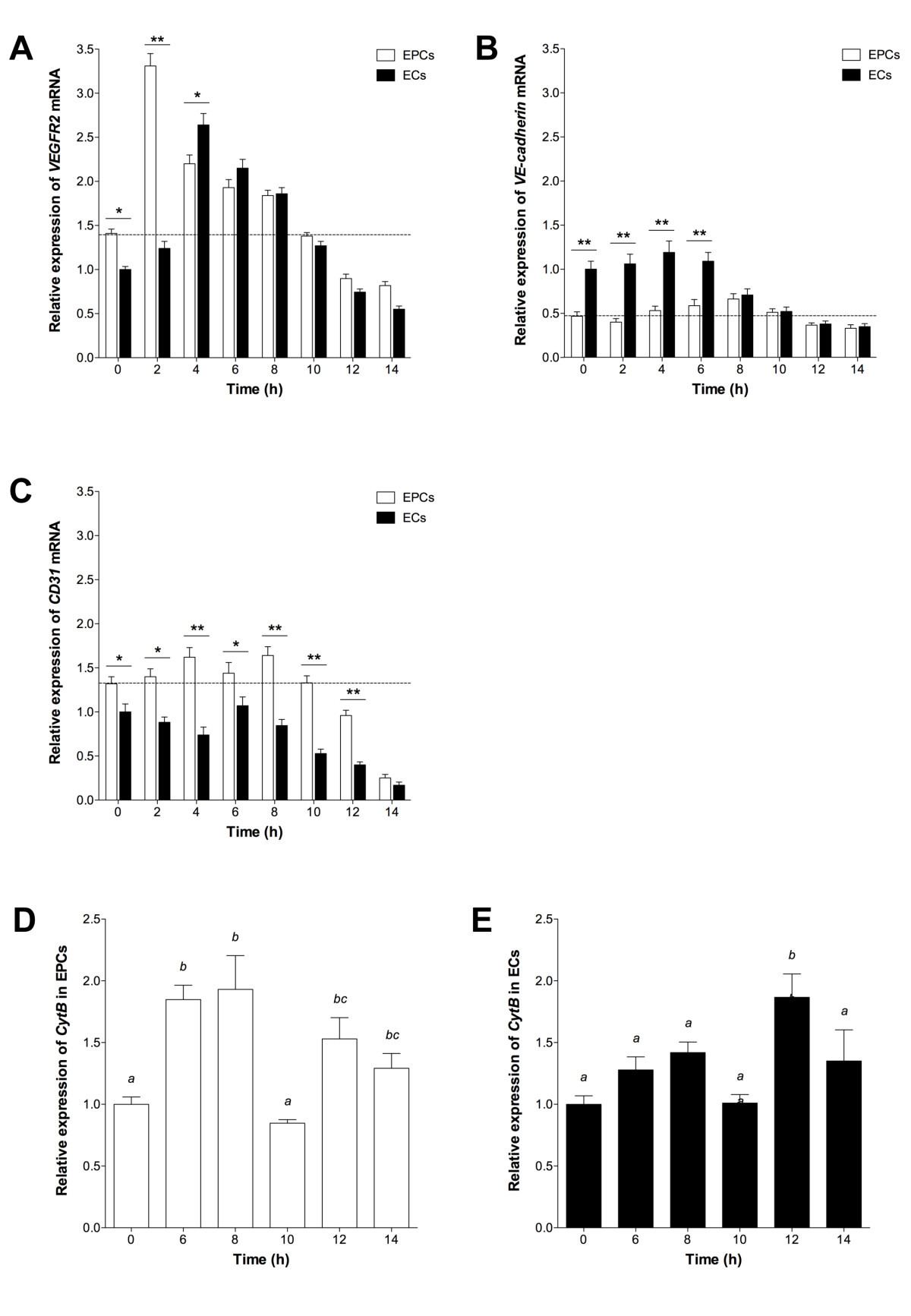
Figure 3
Figure 3 caption
Gene expression during tubule formation. (A) , (B) and (C) mean expression in assayed EPCs and ECs, relative to 60% confluent ECs (± SEM; n = 3). Dotted line indicates expression in 60% confluent EPCs. Significant differences between cell types indicated (*P < 0.05, **P < 0.01). CytB expression in (D) EPCs and (E) ECs, relative to 0 h. Columns with different letters are significantly different (P < 0.05).
In order to confirm that EPCs were able to develop into fully differentiated cells as they contributed to tubule formation, we analysed the number of mitochondrial (mt)DNA copies over the 14 h assay period. This is indicative of mitochondrial number and their ability to generate sufficient levels of ATP. Although EPCs possessed 30% fewer copies of mtDNA at 0 h, they accumulated similar levels to ECs by 6 h and assumed a similar profile from then onwards (data not shown). A similar outcome was observed for the expression of the mtDNA-encoded gene
Derivation of endothelial-like cells from ESCs can by accelerated using EC-conditioned medium
To determine whether ESCs could give rise to endothelial-like cells, we differentiated D3 ESCs over 28 days using spontaneous and directed methods employing the hanging droplet technique.
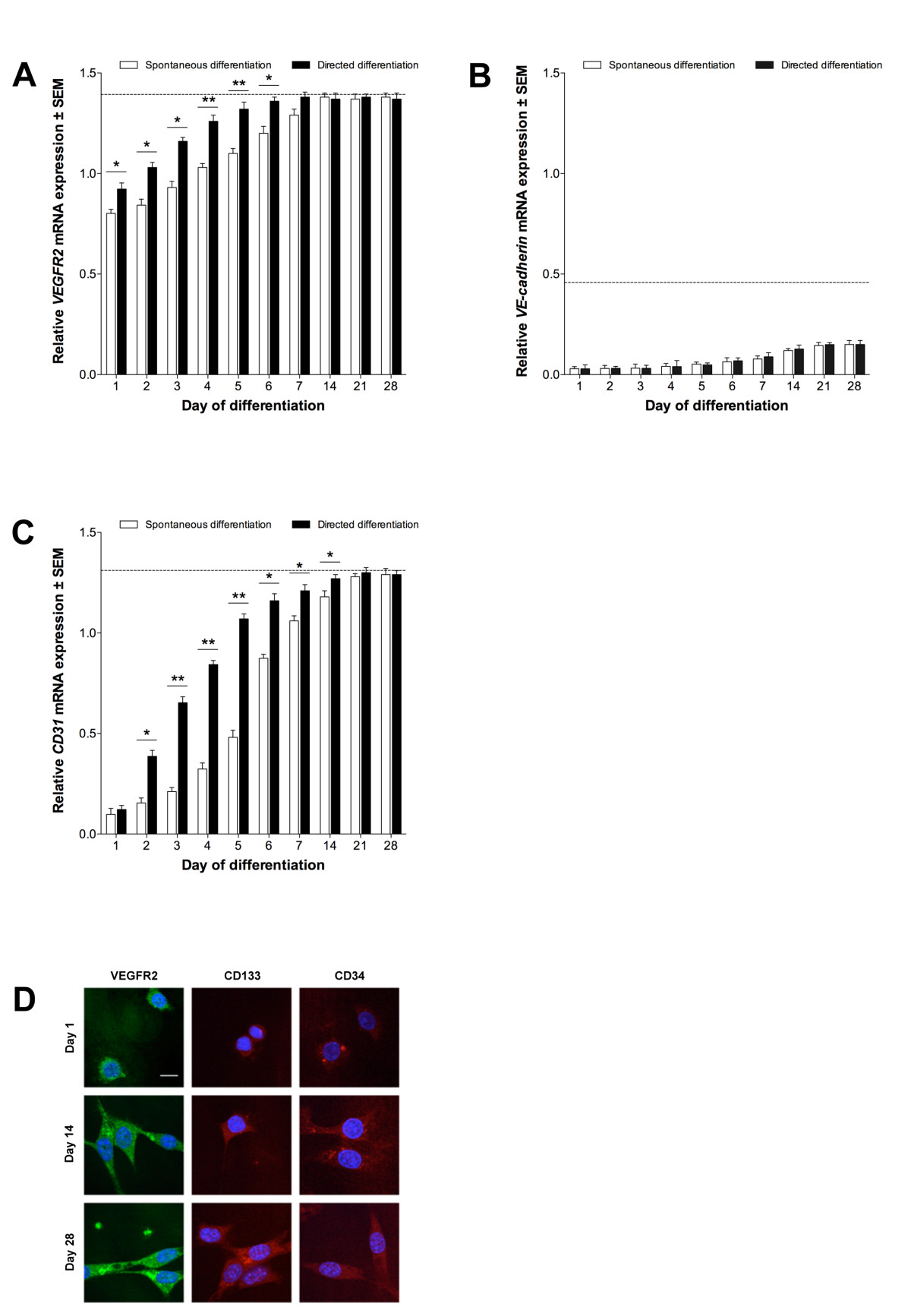
Figure 4
Figure 4 caption
Endothelial differentiation of stem cells. (A) , (B) and (C) expression in ESCs, relative to 60% confluent ECs (± SEM; n = 3). Dotted line indicates expression in 60% confluent EPCs. Directed differentiation with ECCM, significant differences between treatments indicated (*P < 0.05, **P < 0.01). (D): VEGFR2, CD133 and CD34 proteins in ECCM-treated ESCs. Scale bars = 10 μm.
Differentiated ESCs demonstrate angiogenic potential comparable to EPCs
As directed-differentiated ESCs more rapidly adopted patterns of EPC-associated gene expression, we cultured differentiating D7 ESCs on ECMatrix gel to assess their angiogenic potential. The pattern of nodes formed by D7 ESCs was similar to that of both EPCs and ECs (P < 0.05; Figure 5a). To this extent, the number of N1 nodes was maximal at 2 h and decreased throughout the assay. Nevertheless, the number of N2, N3 and N4 nodes increased to their maximum towards the assay's mid point, whilst N5+ nodes were only evident at 8 h. Branch length measurements of D7 ESCs, as for the node data, were similar to EPCs and ECs (P < 0.05; Figure 5b). Mean length increased from 0 h to 6 h, with maximal length observed at 6 h, and no branches were evident by 14 h.
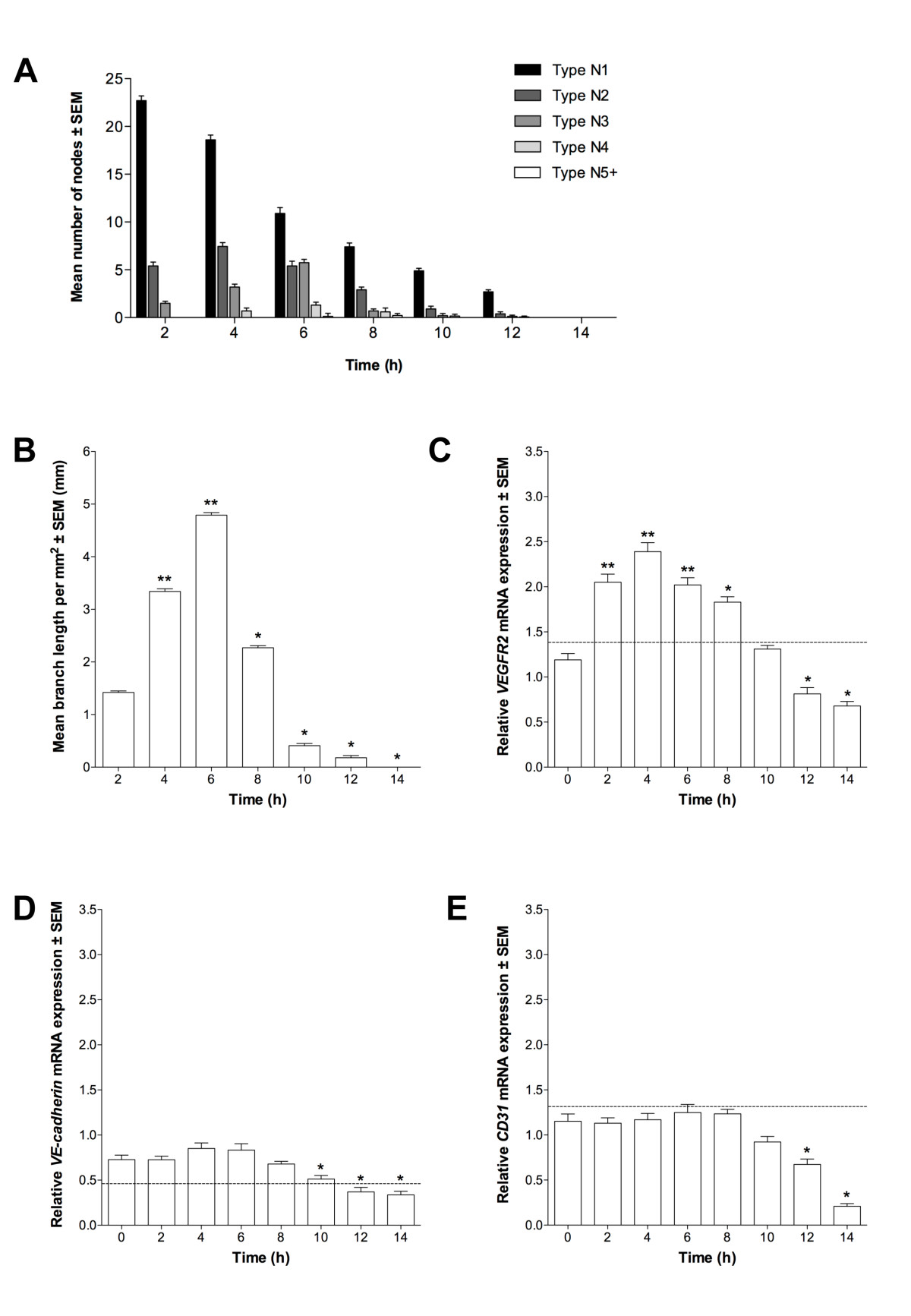
Figure 5
Figure 5 caption
Characterization of differentiating ESCs at D7. Tubule formation as (A) node number ± SEM (n = 3) and (B) tubule branch length ± SEM (n = 3; *P < 0.05, **P < 0.01 vs. 2 h). (C) , (D) and (E) mean expression in D7 ESCs (n = 3) relative to 60% confluent ECs; *P < 0.05, **P < 0.01 vs. 0 h. Dotted line indicates expression in 60% confluent EPCs.
Assayed D7 ESCs were analyzed by qPCR. Expression of
The incorporation of transplanted EPCs into pre-existing endothelial tubules in vitrois dependent on the number of cells transplanted
To determine whether EPC transplantation could enhance the longevity of existing EC tubules, we added EPCs to ECMatrix gel containing branching ECs that had been in culture for 5 h. Transplantation was performed with EPCs equal to 50% or 10% of the original number of ECs. Following transplantation with 10% equivalent EPCs, the greatest number of N1 nodes occurred at 2 h, decreasing by 6 h (Figure 6a). However, there was no significant decrease in N1 nodes between 6 h and 8 h. A progressive decrease in N1 nodes was then seen between 10 h and 12 h, and by 14 h no N1 nodes were present. The maximum number of N2 and N3 nodes occurred at 4 h (1 h prior to transplantation), whilst N4 nodes were maximal at 6 h (1 h after transplantation) and N5+ nodes were only evident at 6 h. Nevertheless, in the 50% EPC-equivalent transplantation assay, it is evident that the number of N1 nodes decreased progressively between 2 h and 8 h (Figure 6b). However, an increase in N1 nodes was seen by 10 h (P < 0.01), and between 10 h and 14 h the mean number of N1 nodes did not change significantly. The greatest number of N2 nodes was recorded at 4 h (1 h prior to transplantation) although N3 nodes were maximal at 8 h (3 h after transplantation). However, the mean number of N4 nodes, first evident at 4 h, increased significantly from 4 h to 6 h (P < 0.05), then decreased progressively between 8 h and 12 h. N5+ nodes were first identified at 6 h, with no significant difference between 6 h and 10 h with a slight reduction between 10 h and 14 h, whilst a significant decrease was observed at 14 h (P < 0.05).
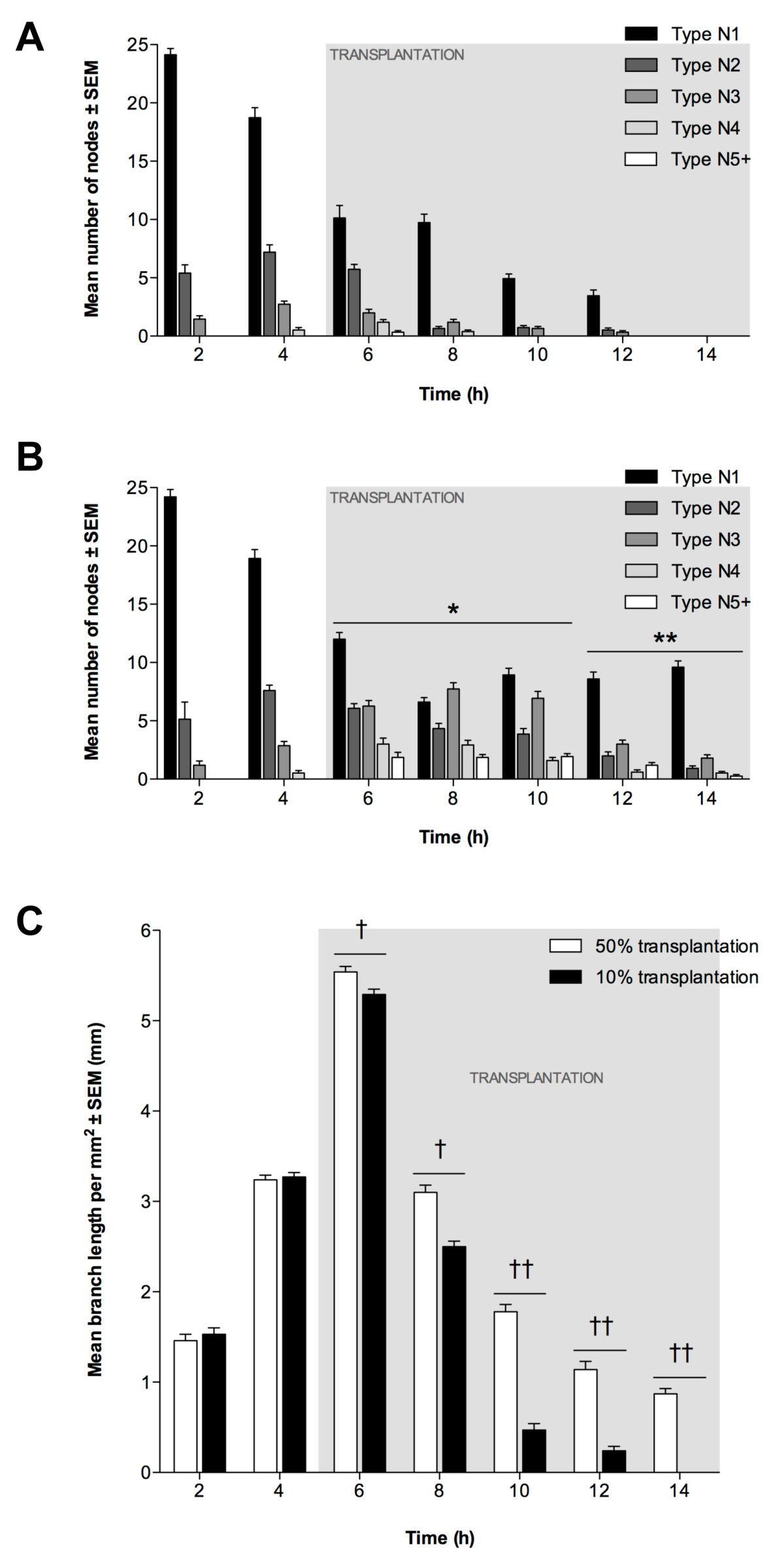
Figure 6
Figure 6 caption
EPC transplantation into EC tubules. Transplantation using 10% (8 × 10) or 50% (4 × 10) EPCs at 5 h. Node counts of (A) 10% and (B) 50% transplantation (± SEM, n = 3). *P < 0.05 and **P < 0.01 vs. 10% EPC transplantation at same time-point. (C): Mean tubule length per mm (±SEM; n = 3). Significant differences between transplantations indicated (†P < 0.05, ††P < 0.01).
Transplantation had a beneficial effect on mean branch length following both 50% and 10% transplantation. It increased from 2 h to 6 h (P < 0.01; Figure 6c) with maximum values for both assays recorded at 6 h, although this was slightly greater for the 50% transplantation (P < 0.05). For both assays, branch length decreased between 6 h and 14 h, with decreases at each time-point being significantly lower following the 10% transplantation (P < 0.01).
By labeling EPCs with fluorescent Qdots prior to transplantation, their localisation into existing EC tubules could be determined. With 50% transplantation, Qdots were detected in high abundance throughout the assay at all time-points (Figure 7a). Whilst Qdots were not detected ubiquitously throughout the existing EC network, between 6 h and 14 h tubule structures were observed, composed entirely of Qdot-labelled EPCs. Following 10% transplantation, Qdots were randomly distributed within the existing EC tubule network (Figure 7b).
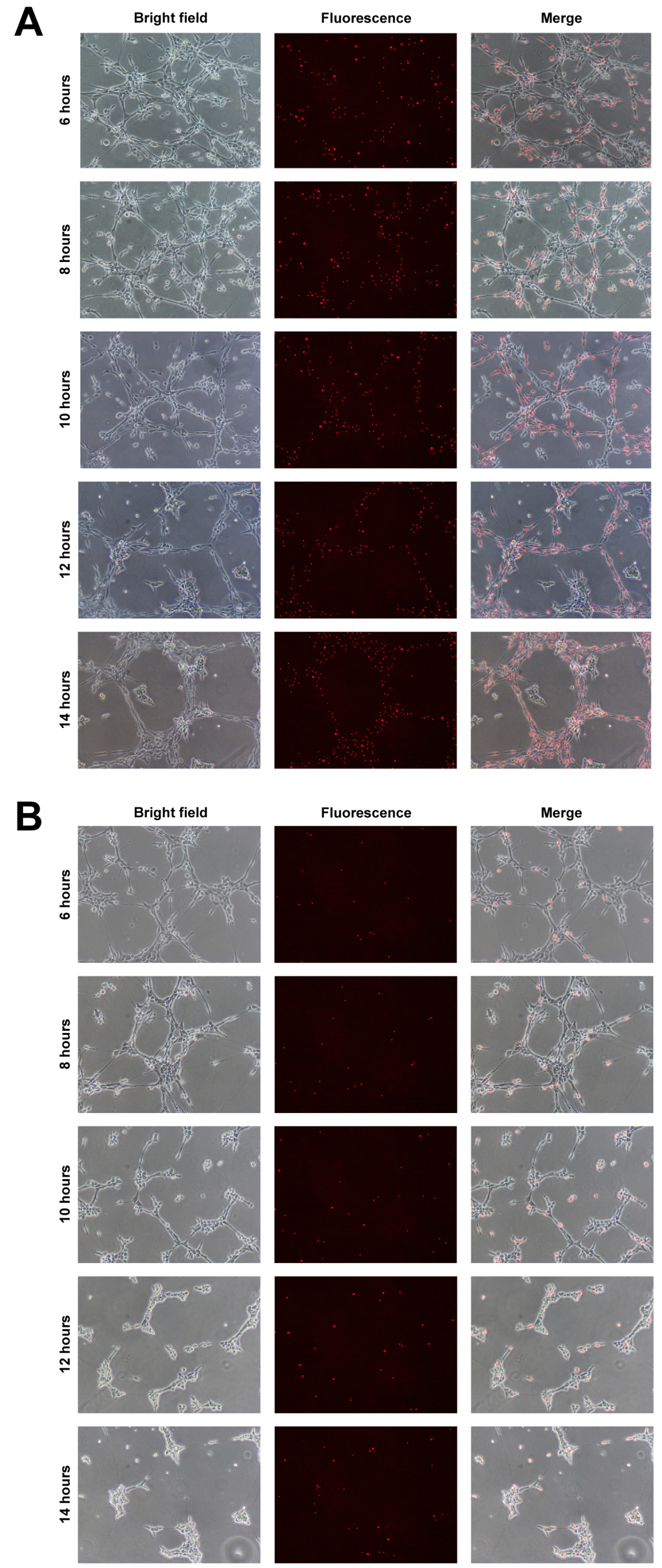
Figure 7
Figure 7 caption
Localisation of EPCs following in vitro transplantation. Transplantation into EC tubules performed using 50% (4 × 104) or 10% (8 × 103) Qdot-labelled EPCs at 5 h. EPC localisation in (A) 50% and (B) 10% transplantation assays, showing bright field, fluorescence and merged images from 6-14 h at 2 h intervals. Scale bars = 500 μm.
The effect of EPC transplantation on expression of VEGFR2, VE-cadherin and CD31 was assessed using qPCR. Prior to transplantation, expression patterns were equivalent to ECs grown on ECMatrix gels without transplantation. Following 50% transplantation, VEGFR2 expression increased significantly compared to non-transplanted ECs (P < 0.05; Figure 8a) but decreased continually following transplantation (6 h) until 14 h. VE-cadherin expression following 50% transplantation was not significantly different to non-transplanted ECs until 8 h when greater expression was detected (P < 0.05; Figure 8b). Although expression decreased from 8 h to 14 h it was still significantly greater at each time-point compared to control ECs (P < 0.05). CD31 expression was not altered significantly by 50% transplantation until 6 h, when greater expression was seen compared to control ECs (P < 0.05; Figure 8c). CD31 expression was greater than non-transplanted ECs until 14 h, at which time no significant difference was observed. 10% EPC transplantation did not result in significant changes in expression of VEGFR2, VE-cadherin or CD31 compared to non-transplanted ECs (P > 0.05).
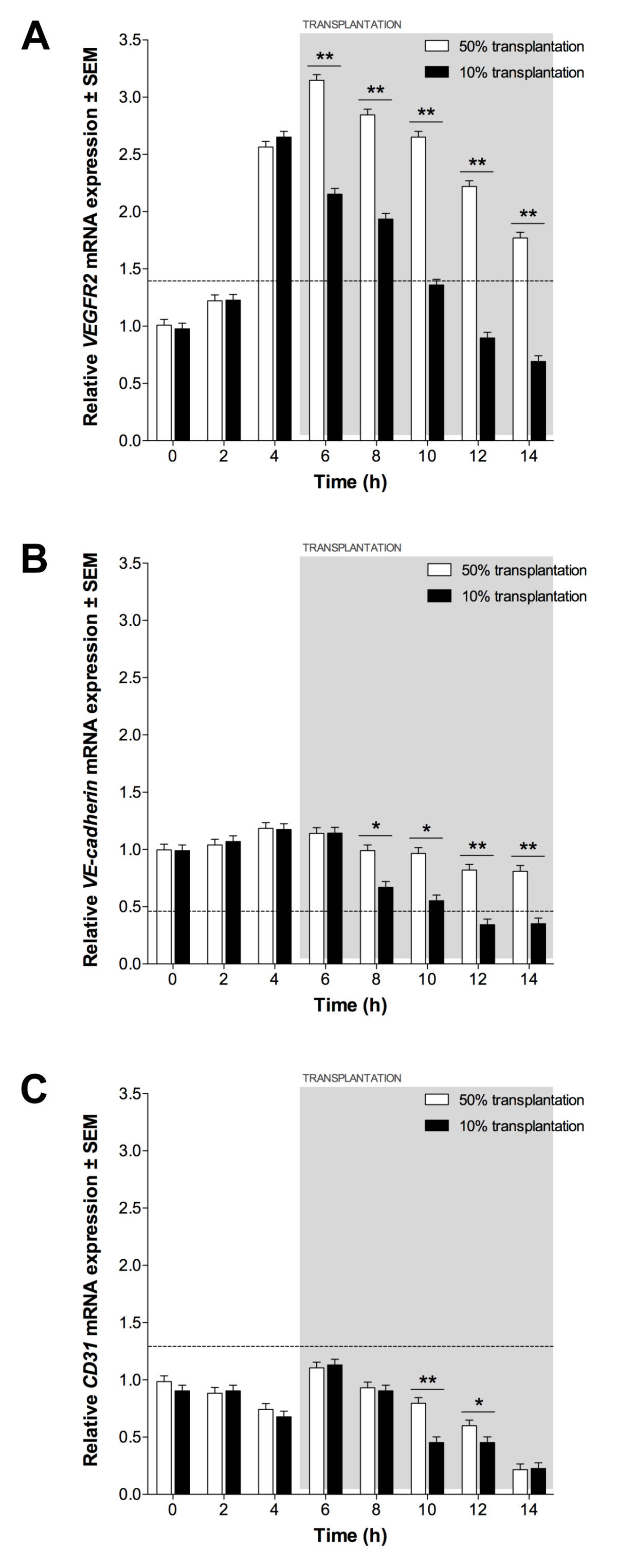
Figure 8
Figure 8 caption
Endothelial expression following EPC transplantation. (A) , (B) and (C) expression in 50% and 10% transplantation assays, relative to expression in 60% confluent ECs. Dotted line indicates expression in 60% confluent EPCs. Significant differences between transplantations indicated (*P < 0.05, **P < 0.01).
Discussion
Differences in endothelial-specific markers were observed between the two stages of endothelial maturation represented by the EPC and EC cell lines, with both mRNA and protein expression confirming their endothelial nature, in agreement with previous studies [8, 17]. When cultured on ECMatrix gel
The effect of EPC transplantation on tubule formation was variable, depending on the relative quantity of cells used. When tubules were transplanted with a quantity of EPCs equivalent to 50% of the number of ECs originally seeded onto the ECMatrix gel, the complexity of the resultant network was significantly different to non-transplanted EC-only controls. To this extent, the number of higher order nodes was greater after 5 h and branch length was increased following transplantation. As well as increasing complexity of the tubule network through the conversion of N1s to more complex structures, such transplantation also increased tubule longevity with nodes of all five types and branches being observed at 14 h, compared to control assays in which the network regressed after 12 h. In contrast, when EC tubules were transplanted with 10% EPCs no significant differences were observed in either node counts or branch lengths, suggesting little effect on either tubule longevity or network complexity.
The increase in tubule longevity seen with 50% EPC transplantation is consistent with previous investigations into the role of circulating EPCs, where they are continually released from the bone marrow to maintain the adult vasculature [34]. In addition to their role in vascular remodelling during angiogenesis, EPCs have also been shown to play an important part in the repair of defects in the EC layers of blood vessels and in the maintenance of vascular wall integrity throughout adult life [35]. The absence of a significant effect on tubule formation with 10% transplantation is interesting because, whilst EPC numbers increase during prolonged angiogenic response, their low number in peripheral blood of healthy adults may illustrate the active support of endothelial cell turnover by EPCs. Nevertheless, aging EPCs exhibit limited regenerative capacity and it may be that regular, low level release of EPCs from bone marrow is more beneficial for continued maintenance of endothelium [36]. Perhaps, if EPCs are indeed involved in subtle vascular maintenance, their effect is too modest to be demonstrated in the tubule formation assay. For this reason, one might not expect 10% EPC transplantation to have a dramatic effect on EC tubules in the assay.
As with the functional readout described above, significant alteration in endothelial-specific gene expression was only observed with 50% EPC transplantation. The effect of EPCs on tubule growth, i.e. the quantitated prolongation of complex nodal structures, is illustrated by greater numbers of higher-order nodes persisting throughout the assay period and the gene expression data. The rate of decrease of
The outcomes of the two transplantation experiments may be representative of two potential
Our data shows that contact inhibition significantly downregulates endothelial-specific gene expression in static EPCs. EPCs intercalating into sites of vessel growth, becoming increasingly contact-inhibited, may have their capacity for neovascularisation affected in this way. If low-level circulating EPCs are subject to relatively low confluency in the bloodstream, the effect of contact inhibition may be reduced. If so, using 60% confluent EPCs (rather than 80% or 100%), as undertaken in our transplantation experiments, may better replicate the microenvironment of EPCs released into the circulation.
Using gene expression analysis, it was established that ESCs demonstrated endothelial phenotypes with different rates of differentiation. Levels of
The action of the ECCM is related to the particular combination of paracrine factors released into the medium during conditioning, such as VEGF and Ang-1 [42, 43]. Furthermore, its effect can be augmented by inclusion of additional factors, such as stem cell factor and erythropoietin, modifying the efficacy of directed differentiation [40]. However, the precise combination of factors necessary for efficient directed differentiation is not yet understood and potentially important factors remain unknown [44]. Consequently, the mechanism controlling the effect of ECCM on cellular expression remains unclear. ESC-derived (D7) EPCs demonstrated
Conclusions
As a transplanted cell population, the angiogenic potential of EPCs is both cumulative and beneficial. We suggest that effective EPC transplantation relies on both the upregulation of the angiogenic phenotype of each individual cell and an increase in cell number overall to produce a significant beneficial effect. Small numbers of transplanted EPCs exhibit limited angiogenic activity in vitro whilst a larger population shows a significant transition in phenotype and a much greater angiogenic potential overall, demonstrating a differential response to vascular maintenance or major injury. We have also demonstrated that cells of a comparable phenotype and angiogenic potential to EPCs, which can be difficult to expand easily in vitro, can be efficiently derived by the differentiation of ESCs. Ultimately, with the proposed therapeutic benefits of EPCs, easily-maintained, ESC-derived EPCs may have potential as a rapidly-expandable and fully characterised alternative to natural EPCs for angiogenic transplantation therapy.
Acknowledgements
This work was funded by a Medical Research Council Grant (GO600273).
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2
Authors’ original file for figure 3
Authors’ original file for figure 4
Authors’ original file for figure 5
Authors’ original file for figure 6
Authors’ original file for figure 7
Authors’ original file for figure 8
References
- Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464-478.
- Vascular progenitor cells and translational research: the role of endothelial and smooth muscle progenitor cells in endogenous arterial remodelling in the adult. Clin Sci. 2009;116:283-299.
- Mechanisms of angiogenesis. Nature. 1997;386:671-674.
- It's all in the blood: circulating endothelial progenitor cells link synovial vascularity with cardiovascular mortality in rheumatoid arthritis?. Arthritis Res Ther. 2005;7:270-272.
- Angiogenesis in skeletal and cardiac muscle. Physiol Rev. 1992;72:369-417.
- Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362-367.
- Intussusceptive angiogenesis--the alternative to capillary sprouting. Mol Aspects Med. 2002;23:S1-27.
- Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med. 2004;8:498-508.
- Endothelial progenitor cells: past, state of the art, and future. J Cell Mol Med. 2004;8:488-497.
- Transplantation of umbilical cord blood-derived endothelial progenitor cells: a promising method of therapeutic revascularisation. Eur J Haematol. 2006;76:1-8.
- Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964-967.
- Circulating endothelial progenitor cells from healthy smokers exhibit impaired functional activities. Atherosclerosis. 2006;187:423-432.
- The role of endothelial progenitor cells in ischemic cerebral and heart diseases. Cell Transplant. 2007;16:273-284.
- Role of progenitor endothelial cells in cardiovascular disease and upcoming therapies. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions. 2007;70:477-484.
- Vascular endothelial growth factor mRNA and protein do not change in parallel during non-inflammatory skeletal muscle ischaemia in rat. The Journal of Physiology. 2006;577:671-678.
- A differential role for nitric oxide in two forms of physiological angiogenesis in mouse. The Journal of Physiology. 2006;570:445-454.
- Circulating endothelial progenitor cells. Br J Cancer. 2005;93:855-858.
- Endothelial Progenitor Cells in Cardiovascular Disorders. Journal of the American College of Cardiology. 2007;49:741-752.
- Human embryonic stem cell (hES) colonies display a higher degree of spontaneous differentiation when passaged at lower densities. In Vitro Cell Dev Biol Anim. 2006;42:54-57.
- Adult stem cells and their trans-differentiation potential-perspectives and therapeutic applications. J Mol Med. 2008.
- Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood. 1996;88:3424-3431.
- Effects of an endothelial cell-conditioned medium on the hematopoietic and endothelial differentiation of embryonic stem cells. Cell Biol Int. 2009;33:1201-1205.
- Differentiation of endothelial cells derived from mouse embryoid bodies: a possible in vitro vasculogenesis model. Toxicology Letters. 2008;180:166-173.
- Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249-1259.
- Human cord blood-derived AC133+ progenitor cells preserve endothelial progenitor characteristics after long term in vitro expansion. PLoS ONE. 2010;5:e9173-.
- Specific Jagged-1 signal from bone marrow microenvironment is required for endothelial progenitor cell development for neovascularization. Circulation. 2008;118:157-165.
- The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis. 2009;12:267-274.
- A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp Cell Res. 2008;314:430-440.
- In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol. 1995;7:862-869.
- Semiautomatic Quantification of Angiogenesis. J Surg Res. 2009.
- Confluence of vascular endothelial cells induces cell cycle exit by inhibiting p42/p44 mitogen-activated protein kinase activity. Molecular and Cellular Biology. 1999;19:2763-2772.
- Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. The Journal of Cell Biology. 2003;161:793-804.
- Inhibition of endothelial cell migration, intercellular communication, and vascular tube formation by thromboxane A(2). J Biol Chem. 1999;274:35562-35570.
- Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis?. J Mol Med. 2004;82:671-677.
- Mathematical modeling of vascular endothelial layer maintenance: the role of endothelial cell division, progenitor cell homing, and telomere shortening. Am J Physiol Heart Circ Physiol. 2004;287:H2651-2658.
- Aging of progenitor cells: limitation for regenerative capacity?. Journal of the American College of Cardiology. 2003;42:2081-2082.
- Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354-3360.
- Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2005;25:296-301.
- Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood. 1996;87:630-641.
- Bone marrow endothelial cell-conditioned medium promotes hematopoietic differentiation of mouse embryonic stem cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2003;11:109-114.
- The homeobox gene HEX regulates proliferation and differentiation of hemangioblasts and endothelial cells during ES cell differentiation. Blood. 2005;105:4590-4597.
- Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. Journal of Cell Science. 2000;113(Pt 1):59-69.
- Differentiation of lymphatic endothelial cells from embryonic stem cells on OP9 stromal cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:2070-2076.
- Paracrine factors of vascular endothelial cells facilitate cardiomyocyte differentiation of mouse embryonic stem cells. Biochemical and Biophysical Research Communications. 2008;377:413-418.

