Citicoline induces angiogenesis improving survival of vascular/human brain microvessel endothelial cells through pathways involving ERK1/2 and insulin receptor substrate-1
Vascular Cell. 2012;
Received: 10 September 2012 | Accepted: 27 November 2012 | Published: 10 December 2012
Vascular Cell ISSN: 2045-824X
Abstract
Background
Citicoline is one of the neuroprotective agents that have been used as a therapy in stroke patients. There is limited published data describing the mechanisms through which it acts.
Methods
We used
Results
Citicoline presented no mitogenic and chemotactic effects on hCMEC/D3; however, it significantly increased wound recovery, the formation of tube-like structures in Matrigel™ and enhanced spheroid development and sprouting. Citicoline induced the expression of phospho-extracellular-signal regulated kinase (ERK)-1/2. Kinexus assays showed an over-expression of insulin receptor substrate-1 (IRS-1). Knock-down of IRS-1 with targeted siRNA in our hCMEC/D3 inhibited the pro-angiogenic effects of citicoline. The percentage of surviving cells was higher in the presence of citicoline. Citicoline treatment significantly increased the numbers of new, active CD105-positive microvessels following MCAO.
Conclusions
The findings demonstrate both a pro-angiogenic and protective effect of citicoline on hCMEC/D3
Keywords
Citicoline Angiogenesis Apoptosis Stroke Ischaemia IRS-1Background
Citicoline, is an essential component of cell membrane phospholipids, and is one of the neuroprotective agents that have been used as a therapy in stroke patients. It has been extensively tested in many stroke studies and has shown promising results with regard to the reduction of infarct size and improvement of functional recovery. Citicoline (CDP-choline or cytidinediphosphate choline; cytidine 5′-diphosphocholine) is a complex organic molecule composed of ribose, pyrophosphate, cytosine and choline [1]. Citicoline has been suggested to provide beneficial recovery and neuroprotective effects in brain traumatic injuries, stroke, brain ageing and neurodegenerative diseases [2, 3], although the exact mechanisms through which it operates are not fully understood.
The most recent clinical and pre-clinical updates have been published by Davalos and Secades [4]. In this article, they provide information on basic research studies using animal models, where individual studies have demonstrated for example, increased protection against cognitive impairment in chronic hypoperfused rats [5], as well as a meta-analysis of the effects of citicoline using a systematic review of all the data collected (15 studies on focal ischaemia using the rat model). Here, Giralt et al. [6] showed overall a reduced infarct volume of 30% (transient occlusion) and 25% (permanent occlusion), however disappointingly, there was no improvement in neurological outcome. Finally, Saver et al., [7] performed a meta-analysis using 10 trials of ischaemic/haemorrhagic stroke where the patients were treated with citicoline. Results showed that citicoline treatment was associated with a significant reduction in the frequency of death and disability at long-term follow up with no adverse affects. In all instances, citicoline was administered within 24 h of the stroke occurrence. Interestingly, ‘priming’ of patients with citicoline following transient ischaemic attack (TIA) or minor stroke has not been considered as yet and also, since the mechanism of its action is not fully elucidated, optimal application cannot be determined accurately.
In terms of analysis of the signalling mechanisms associated with citicoline-induced protection, Krupinski et al., [8] showed that citicoline treated animals showed a dramatic reduction in immunoreactive cells for pro-caspases 1, 2, 3, 6 and 8 in the ischemic infarction area when compared with the control group. The number of cells expressing cleaved caspase-3 and nuclear DNA fragmentation in the penumbra area was significantly reduced in animals treated with citicoline. The data suggests that citicoline may protect the ischemic neurons by providing a negative effect on the activation of the caspase apoptotic pathway.
As far as we are aware, no-one has examined the possible beneficial effects of citicoline treatment on revascularization and angiogenesis after stroke. Our preliminary
Methods
Cell culture
Human brain micro-vessel endothelial cell (EC) line named hCMEC/D3 was grown in endothelial basal medium-2 (EBM-2) medium supplemented with growth factors and hydrocortisone as described previously [9]. Cells were seeded into T25 flasks pre-coated with 0.1% collagen and maintained in a humidified 5% CO2 atmosphere at 37°C. Every three days reaching the confluence, the cells were detached under the enzymatic activity of the trypsin then the cells in suspension were centrifuged for 5 min at 1300 rpm then seeded into new pre-coated T25 flasks. Throughout the study, the cells used were between passages 28 and 38. Cells were routinely cultured as described in our previously published work [10].
Staurosporin/ionophore-induced apoptosis assay
In this assay, glass coverslips were sterilized in a bath of 100% ethanol for 10–20 min then left to air dry. The coverslips were put in a 24-well plate and pre-coated with 500 μl of 0.1% collagen in acetic acid and then incubated for 1 h. hCMEC/D3 were cultured in complete medium at a concentration of 5 × 104 cells/ml on collagen pre-coated coverslips for 4 h-incubation. Then, the medium was replaced with serum-free medium and the cells were incubated. After 24 h incubation, the cells were pre-incubated with 10 μM citicoline for 4 h prior to apoptosis induction. After the pre-incubation with citicoline, apoptosis was induced using calcium ionophore (10 μM/24 h); or staurosporin: 10 μM/4 h (concentrations determined from pilot studies as optimal) or by exposure to oxygen-deprivation (12 h, 1% O2; hypoxia confirmed by up-regulation of HIF-1α as determined in our pilot studies). These concentrations have previously been shown to induce apoptosis in about 40-90% of the cell population. For staining, one hour before the termination of the experiment, propidium iodide (PI; 10 μg/ml) was added in each well as an indicator of DNA damage. After 1 h, the medium was discarded and the cells were washed with PBS then fixed with 4% paraformaldehyde for 20 min at room temperature. Subsequently, cells were washed three times with PBS and exposed to 1 μg/ml Hoechst 6024 stain solution diluted in PBS at room temperature for 30 min. Finally, the cells were washed three times with PBS and one drop of FluorSave™ reagent was added on frosted glass slides and the coverslips were put upside down on the drops. An average of six fields at x200 of magnification was photographed per coverslip using an Axivoert fluorescence microscope. The apoptotic index is expressed as the number of apoptotic cells relative to the total number of cells (% apoptotic cells). In this experiment, triplicate wells were run for each condition with controls consisting of untreated cells.
Angiogenesis assays: cell proliferation
hCMEC/D3 cells were seeded at a concentration of 8 × 104 cells/ml in 500 μl of complete basal medium in each well of 24-well plate. After 4 h, the medium was changed to serum-poor medium (SPM) containing 1% FBS containing different concentrations of citicoline (1 μM, 10 μM and 100 μM; NOTE; pilot experiments were carried out using 1-100 μM citicoline and optimized for the use of 10 μM subsequently as this produced the most prominent responses). After 72 h incubation, cells were washed with PBS and detached with trypsin. Cells were counted in a Coulter counter at least three times for each well. In this experiment, cells were treated in triplicate for each experimental condition. Cell migration (Boyden Chamber: chemotaxis assay)- Inserts of Transwell Costar® porous membranes were coated with 0.1% collagen and left for air dry in a 24-well plate. (Wound recovery)-cells were seeded onto plastic coverslips and when confluent, were scraped with a razor to induce straight lesions. Cells were seeded in 100 μL of serum- poor medium containing 1% FBS at a concentration of 7.3 × 104 cells/ml. The inserts containing hCMEC/D3 were placed in a 24-well plate containing 500 μl of serum poor medium containing 1% FBS and supplemented with either citicoline (10 μM/ml) or FGF-2 (25 ng/ml), used as a positive control. Cells were treated in triplicate for each experimental condition. (Boyden chamber)-After 24 h incubation, the medium was removed from the insert and the cells which had migrated through the pores to the bottom side of the insert were fixed with 4% paraformaldahyde. Cotton swabs were soaked with PBS and used in order to remove the cells that did not migrate. After fixation, the migrated cells were stained with Giemsa (3 minutes). An optical microscope was used to count randomly five microscopic fields from each insert. (Wound healing)-after 24 h the plastic coverslips were fixed in paraformaldehyde and cell migration examined under a microscope at low power. Tube-like structure formation in Matrigel- EC (2 × 106 cells/ml) were mixed with equal volume of growth factor-reduced Matrigel™ containing citicoline (10 mM) or fibroblast growth factor-2 (FGF-2) (25 ng/ml). Any material in the contact with the gel was cold to avoid the gel polymerization. In a 48-well plate, a total amount of 35 μl of this mixture was placed in a tear-like drop in the middle of the well and left to polymerize for 1 h incubation. Then, 0,5 ml of complete medium was added into each well. After 24 h incubation, 4% paraformaldahyde was added to fix the endothelial tube-like structures embedded in the gel. Five areas from each well were counted under optical microscope. Spheroids were produced by inoculation of centrifuged endothelial cells into matrigel with or without addition of citicoline or FGF-2 as a positive control and their development including sprout formation monitored as described above over a period of 7 days. At least 10 sprouts were measured for each condition and the experiments repeated at least 3 times.
Protein extraction and western blotting
EC (3 × 105 cells/ml; 2 ml) were seeded in complete medium in 6-well plate and incubated for 48 h. Then, the medium was replaced with SPM containing 1% FBS. After 24 h incubation, the cells were stimulated with citicoline at 1-10-50 μM for 10 min then the cells were immediately washed with 1 ml of PBS and gently lysed on ice in 50 μl of ice-cold homogenized lysis buffer (PH 7.2). The cells then were scraped, the cell lysate proteins collected and transferred into 0.5 ml micro-centrifuge tubes. Samples were sonicated four times for 10 seconds each time with 10–15 second intervals on ice to rapture the cells and to shear nuclear DNA. Cell lysates were centrifuged at 20,000 g for 20 min and the protein concentration was determined using the BioRad protein assay.
In our preliminary un-published studies, we showed that citicoline also strongly induced angiogenesis in our bovine aortic EC (BAEC) and for this reason we performed a phospho-site protein expression screen using Kinex™ antibodies microarray KAM1.3 and performed by Kinexus (Bioinformatics Corporation, Vancouver, Canada). The most relevant phospho-protein expression up- or down-regulated by citicoline were confirmed by Western blotting (IRS-1, HER2 and Histone H2B; see additionally supplied data). Since phosphorylation of IRS-1 was most apparent when translated to our hCMEC/D3 cells, we investigated its role in intracellular signalling in more detail here. Standard siRNA transfection (using lipofectamine) was employed to induce a transient down-regulation of IRS-1 RNA (approximately 85-90%-data not included; Figure 1B and C). After 24 h exposure to siRNA or scrambled sequences, cells were used for analysis of tube-like structure formation described previously. The experiment was repeated twice in triplicate wells and a representative example shown.
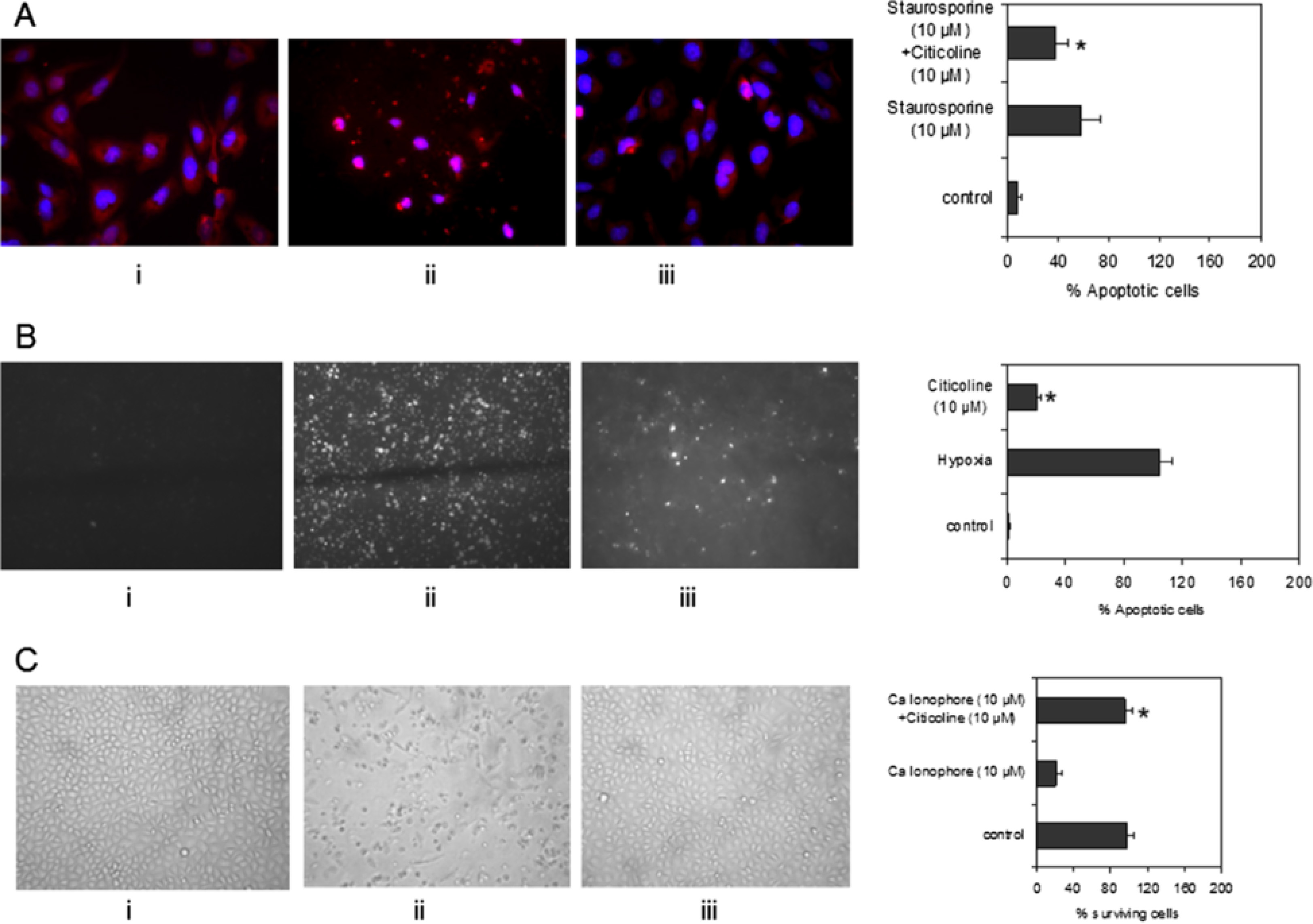
Figure 1
Figure 1 caption
Effect of citicoline, hypoxia, and the Ca ionophore A23187 on cell death in hCMEC/D3. A) Shows control hCMEC/D3 without citicoline or staurosporin treatment (i), cells treated with staurosporin (ii; 10 μM), and cells pre-incubated with citicoline for 4 h before apoptosis was induced with 10 μM staurosporin (24 h; iii). The presence of citicoline (10 μM) was sufficient to significantly inhibit cell apoptosis (iii) B) Shows control hCMEC/D3 without citicoline or hypoxia treatment (i), cells treated after exposure to hypoxia (ii; 12 h; 1% O) and cells pre-incubated with citicoline for 4 h prior to subjecting to hypoxia (iii). The data shows that citicoline (10 μM) was able to significantly reduce the number of cells undergoing apoptosis (iii). C) Shows that citicoline protected the cells against apoptosis induced by calcium ionophore. (i) Shows control cells, (ii) the effect of 10 μM/24 h treatment with Ca ionophore, and (iii), the effect of 4 h pre-treatment with citicoline before addition of 10 μM Ca ionophore. Citicoline significantly protected against apoptosis induced by the ionophore. The bar graphs shows data from one representative experiment carried out in triplicate wells. All experiments were performed three times. Cells were considered apoptotic when cell nuclei demonstrated positive PI/Hoechst staining and apoptotic morphology (Data not shown). For quantification of PI/Hoechst -positive cells, four fields per section were examined at 200-fold magnification. The apoptotic index was calculated using the formula: Apoptotic index = 100 * (number of PI/Hoescht + cell nuclei per field/total number of cell nuclei per field).
Transient rat MCAO
Four male Wistar rats (Charles River) were used in each group (control and citicoline treated), with a weight of 280-320 g at the beginning of the study. Animals were kept on a 12/12 hours light/darkness cycle, with access to food and water
The ischemic lesion was induced by transient (90 minutes) occlusion of the right middle cerebral artery (MCAO), using the intraluminal thread occlusion method, described elsewhere [11], under isofluorane anesthesia. The occlusion period and the successful reperfusion of the right MCA were controlled by continuous recordings of ipsilateral laser-Doppler flowmetry (LDF). Animals were intraperitoneally treated with either citicoline (1000 mg/Kg) or saline. Drugs were daily administered until day 7. The first dose was given 15 minutes before reperfusion. 21 days after occlusion animals were anesthetized and perfused through the heart with heparinized saline, followed by 4% paraformaldehyde. Brains were cryoprotected with 30% sucrose, frozen at −40°C and 8-μm thick coronal sections were cut in a cryostat. 1, 7 and 21 days after occlusion rats were anesthetized and MRI T2-weighted images were acquired at 7 T (Bruker BioSpin), in order to study evolution of lesion volume. There was no mortality in this experimental group. Procedures followed were in accordance with institutional guidelines.
Immunohistochemistry
Immunohistochemical staining was used to determine the relative numbers of microvessels (anti-CD31) and active vessels (anti-CD105) in control and citicoline treated stroked brain tissue after 21 days (n = 5). The mean number of vessels from five areas of stroke-affected tissue was counted microscopically (× 20) in eight sections per animal. Double immunofluorescence was used to assess the distribution of the phospho-protein IRS-1 in relation to active microvessels in the stroke region (CD105/endoglin mouse monoclonal antibody). After incubation with primary antibodies for 1 h at room temperature (1:100), sections were washed and then incubated with the appropriate secondary antibodies (1:50) − fluorescein isothiocyanate-conjugated sheep anti-mouse IgG (Jackson) or tetramethylrhodamineisothiocyanate-conjugated rabbit anti-goat (Jackson). Images were captured with Nikon 80i Digital Microscope using Nis Elements 3.21 software with multichannel capture option. Negative control slides were included where the primary antibody was replaced with PBS.
Statistical analysis
All
Results
Citicoline protects hCMEC/D3 against cell damage/apoptosis
EC were pre-incubated with 10 μM citicoline for 4 h. Then, the cells were incubated with 1-10 μM staurosporin in DMSO for 4 h and 24 h, respectively. These concentrations were chosen in order to identify appropriate levels of cell damage/apoptosis and were classified as preliminary studies. In these experiments, the numbers of apoptotic cells were approximately 40% and 60% at 10 μM (4 h and 24 h respectively) (data not shown). Therefore, 10 μM/4 h was the chosen treatment in order to evaluate whether citicoline could protect the cells against cell death pathways. Pre-treatment of hCMEC/D3 cells with citicoline significantly decreased the number of damaged/apoptotic cells, determined by counting the numbers of PI-positive nuclei at the end of the experiment, under these conditions (Figure 1A; P < 0.05). Since hypoxia is a key feature of acute ischaemic stroke and a major activator of cell apoptotic pathways, we examined its effect on our EC in the presence of citicoline (10 μM/4 h pre-incubation). Again, the presence of citicoline in the media significantly protected the cells against apoptosis/cell damage (Figure 1B; p < 0.05). Increased expression of and release of Ca2+ Kainic acid and glutamate also leads to increased cell death after stroke. Ionophore treatment of EC is known to induce Ca influx and rapid dephosphorylation of eNOS at Thr495 resulting in eNOS activation. For this reason, we exposed our EC to the Ca ionophore A23187 (10 μM/24 h), after pre-treatment with citicoline. Figure 1C shows that the Ca ionophore caused significant reduction in survival of cells after 24 h. However in the presence of citicoline, the effects were reversed and the cells remained attached and morphologically identical to control-untreated cells (p < 0.05). The possible mechanism through which citicoline provides this protection is discussed later in this results section.
Discussion
In this study, we have, for the first time, demonstrated both a vascular protective, and pro-angiogenic effect of citicoline using
In addition, although citicoline had no effect on the chemotaxis of HBMEC/D3 determined by using the Boyden chamber method it significantly increased the number of migrating cells in the scratch wound healing assay. Citicoline also significantly increased the formation of tube-like structure in Matrigel producing a stronger effect than the known mitogenic factor FGF-2 (p <0.05). Although citicoline had no mitogenic and chemotactic effects on EC, it had a significant effect on cell differentiation and migration which are two of the key steps of the angiogenic process. This may be an extremely valuable novel finding in regard to understanding the potential mechanisms through which citicoline treatment results in patient recovery, since both protection of EC and induction and maintenance of angiogenesis is key to both short-term and chronic re-vascularization after stroke impacting indirectly but significantly also on neuronal survival and re-integration [19].
Western blotting demonstrated that citicoline induced pERK1/2 expression, a key mitogenic signalling protein known to be involved in angiogenesis and generally stimulated by growth factors through interaction with their receptors [20]. This data demonstrated the potential of citicoline to activate intra-cellular signal transduction pathways and induce phosphorylation of down-stream angiogenic molecules; hence we investigated this ability in more detail by analysis of the Kinexus-phospho-protein Western screen following treatment of vascular EC with citicoline. Interestingly, treatment with citicoline modified the expression of only several of the >500 proteins on the array showing a degree of specificity. IRS-1 and Her2 were both phosphorylated in the presence of citicoline. These two proteins have not been implicated in stroke recovery pathways or stroke angiogenesis until now.
Here, we went on to demonstrate the importance of IRS-1 in mediating the angiogenic effects of citicoline
Only recently, IRS-1 over-expression was attributed to increased angiogenesis in human EC in association with increased Akt and VEGF-A expression [12], whilst
A further interesting finding was the up-regulation of HER2 by citicoline in our vascular ECs
Conclusion
In conclusion, citicoline induces angiogenesis and improves survival of human brain microvessel endothelial cells through pathways involving p-ERK1/2, and IRS-1 and it is probable that other novel signalling intermediates are also involved including Histone H2B and HER2 (more studies are needed to confirm this) and therefore following chronic treatment, its beneficial effects after stroke may in part be due to revascularization. Based on our findings, optimization of its therapeutic use to include vascular tissue regeneration should be re-considered.
Disclosures
Authors have no additional disclosures.
Sources of funding
This study was supported from research grant from Ferrer International.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2
Authors’ original file for figure 3
Authors’ original file for figure 4
Authors’ original file for figure 5
Authors’ original file for figure 6
References
- Probably role of citicoline in stroke rehabilitation: review of the literature. Rev Neurol. 2012;54(3):173-179.
- Clinical use of cholinomimetic agents: a review. J Head Trauma Rehabil. 2002;17:314-321.
- A chronic treatmente with CDP-choline improves functional recovery and increases neuronal plasticity after experimental stroke. Neurobiol Dis. 2007;26:105-111.
- Citicoline preclinical and clinical update 2009–2010. Stroke. 2011;42:S36-S39.
- Citicoline protects against cognitive impairment in a rat model of chronic cerebral hypoperfusion. J Clin Neurol. 2009;5:33-38.
- Selecting the optimal dose of citicoline treatment in animal models of focal cerebral ischaemia through a meta-analysis. Cerebrovasc Dis. 2010;29:165-.
- Citicoline: updata on a promising and widely available agent for neuroprotection and neurorepair. Rev Neurol Dis. 2008;5:167-177.
- CDP-choline reduces pro-caspase and cleaved caspase-3 expression, nuclear DNA fragmentation, and specific PARP-cleaved products of caspase activation following middle cerebral artery occlusion in the rat. Neuropharmacology. 2002;42:846-854.
- Blood–brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872-1874.
- Modified C-reactive protein is expressed by stroke neovessels and is a potent activator of angiogenesis in vitro. Brain Pathol. 2010;20:151-165.
- Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84-91.
- Potent in vivo antiangiogenic effects of GS-101 (5′-TATCCGGAGGGCTCGCCATGCTGCT-3′) an antisense oligonucleotide preventing the expression of insulin receptor substrate-1. J Pharmacol Exp Ther. 2009;329:496-504.
- CDP-choline protects motor neurons against apoptotic changes in a model of chronic glutamate excitotoxicity in vitro. Folia Neuropathol. 2008;46:139-148.
- Effects of citicoline used alone and in combination with mild hypothermia on apoptosis induced by focal cerebral ischaemia in rats. J Clin Neurosci. 2010;17:227-231.
- Neuroprotective effect of citicoline on retinal cell damage induced by kainic acid in rats. Korean J Ophthalmol. 2005;19:219-226.
- Molecular mechanisms involved in farnesol-induced apoptosis. Cancer Lett. 2010;287:123-135.
- Competetive inhibition of choline phosphotransferase by geranylgeraniol and farnesol inhibits phosphatidylcholine synthesis and induces apoptosis in human lung adenocarcinoma A549 cells. J Biol Chem. 1998;273:26179-26186.
- Apoptotic phosphorylation of histone H2B by mammalian sterile twenty kinase. Cell. 2003;113:507-517.
- Pathophysiology of acute ischaemic stroke: an analysis of common signalling mechanisms and identification of new molecular targets. Pathobiol. 2006;73:159-175.
- Can angiogenesis be exploited to improve stroke outcome? Mechanisms and therapeutic potential. Clin Sci. 2006;111:171-183.
- Down-regulation of IRS-1 expression causes inhibition of corneal angiogenesis. Invest Ophthalmol Vis Sci. 2005;46:4072-4078.
- Tolerability and safety of GS-101 eye drops, an antisense oligonucleotide to insulin receptor substrate-1: a first in man phase 1 investigation. Br J Clin Pharmacol. 2009;68:169-173.
- Combination treatment with HER-2 and VEGF peptide mimics induces potent anti-tumour and anti-angiogenic responses in vitro and in vivo. J Biol Chem. 2011;286:13626-13637.
- HER2 signaling modulates the equilibrium between pro-and antiangiogenic factors via distinct pathways: implications for HER2 –targeted antibody therapy. Oncogene. 2006;25:6986-6996.

