The role of angiogenesis in the pathology of multiple sclerosis
Vascular Cell. 2014;
Received: 9 September 2014 | Accepted: 6 November 2014 | Published: 28 November 2014
Vascular Cell ISSN: 2045-824X
Abstract
Angiogenesis, or the growth of new blood vessels from existing vasculature, is critical for the proper development of many organs. This process is inhibited and tightly regulated in adults, once endothelial cells have acquired organ-specific properties. Within the central nervous system (CNS), angiogenesis and acquisition of blood–brain barrier (BBB) properties by endothelial cells is essential for CNS function. However, the role of angiogenesis in CNS pathologies associated with impaired barrier function remains unclear. Although vessel abnormalities characterized by abnormal barrier function are well documented in multiple sclerosis (MS), a demyelinating disease of the CNS resulting from an immune cell attack on oligodendrocytes, histological analysis of human MS samples has shown that angiogenesis is prevalent in and around the demyelinating plaques. Experiments using an animal model that mimics several features of human MS, Experimental Autoimmune Encephalomyelitis (EAE), have confirmed these human pathological findings and shed new light on the contribution of pre-symptomatic angiogenesis to disease progression. The CNS-infiltrating inflammatory cells that are a hallmark of both MS and EAE secrete several factors that not only contribute to exacerbating the inflammatory process but also promote and stimulate angiogenesis. Moreover, chemical or biological inhibitors that directly or indirectly block angiogenesis provide clinical benefits for disease progression. While the precise mechanism of action for these inhibitors is unknown, preventing pathological angiogenesis during EAE progression holds great promise for developing effective treatment strategies for human MS.
Keywords
Multiple sclerosis Angiogenesis Hypoxia Experimental autoimmune encephalomyelitis Blood–brain barrier Hypoxia VEGF Endothelial cellIntroduction
Multiple sclerosis is a chronic inflammatory disease of the CNS, whose hallmarks include blood–brain barrier (BBB) breakdown as well as CNS inflammatory infiltration, demyelination and eventual axonal destruction. Approximately 2.3 million people worldwide suffer from MS and its debilitating symptoms, which range from numbness and tingling to more severe examples of partial or complete paralysis [1]. Experimental autoimmune encephalomyelitis (EAE), one of several animal models used to study MS, recapitulates many inflammatory and demyelinating characteristics of the disease [2],[3]. In this paradigm, immunizing mice with myelin protein plus complete adjuvant results in a T cell-mediated disease displaying CNS inflammatory infiltration, BBB leakage and demyelination [2].
Angiogenesis, or the sprouting of new blood vessels from existing vasculature, is most prevalent in rodents and humans during development, and is generally inactive in adults except under certain regulated conditions such as wound healing and during female reproductive cycles [4]. Within the CNS, the process of angiogenesis is integrated with a series of programmed changes in endothelial cells, which culminates in the formation of a tight barrier [5]. The key features of this barrier include tight junctions, low levels of transcytosis and transporters for specific molecules, which together ensure a selectively permeable barrier that maintains CNS homeostasis and protects the tissue from intrusion by unwanted molecules, ions and cells [6],[7]. Several cell types and signaling pathways that regulate developmental CNS angiogenesis and BBB formation have been described [8]-[12]. While there is increasing evidence that angiogenesis occurs in CNS diseases with impaired barrier function such as stroke [13] or MS [14],[15], the role of angiogenesis in human MS pathology remains unclear. Here we highlight in a brief review recent studies suggesting that angiogenesis, stimulated by the presence of invading immune cells, plays a role in both the progression and severity of human MS.
Timing of angiogenesis in the EAE model and its relevance to recovery
Blood vessel abnormalities such as impaired barrier function have long been associated with MS lesions [16]; however only recently has there been evidence confirming both the presence of angiogenesis in MS patients as well as establishing its onset in relation to disease progression. Angiogenesis, as measured by an increase in vessel number and size, was first described to be present not only within and at the edge of acute MS lesions but also in the area surrounding the plaque, where it is often associated with areas of inflammation [15]. In further support of these findings, Proescholdt
Although pathological studies in human MS tissue have demonstrated that angiogenesis occurs in MS lesions [14], determining when this process begins in patients has been challenging. Recently, magnetic resonance imaging (MRI) studies on cerebral perfusion differences in MS patients have begun to shed light on when angiogenesis begins in relation to disease progression. This method has proven effective in assessing whether angiogenesis is present, as established by correlation between increased cerebral perfusion and histologically determined vessel density [19]. Using MRI to examine cerebral perfusion of gadolinium-enhancing lesions, Wuerfel
In the EAE mouse model for MS, angiogenesis has been confirmed histologically in many areas with clear inflammation and demyelinating lesions [22]-[25]. Boroujerdi
While the timing of angiogenesis during disease progression in MS and EAE is still under investigation, its presence in both forms of the disease raises the question whether angiogenesis is beneficial or detrimental to clinical recovery. The majority of current studies would suggest that angiogenesis is detrimental to MS pathogenesis [18],[23],[24],[26]. The pre-symptomatic increase in angiogenesis observed by Boroujerdi
Are inflammatory cell infiltration and angiogenesis linked?
Several factors contribute to angiogenesis in EAE and MS lesions; it is known that a state of hypoxia exists in active MS lesions. Such hypoxic regions are due to numerous changes in the local lesion environment. Lassmann
Hypoxic conditions within the CNS also modulate immune cell responses. Activated T cells that cross the BBB into this locally hypoxic CNS environment shift their profile of cytokine expression. Mor
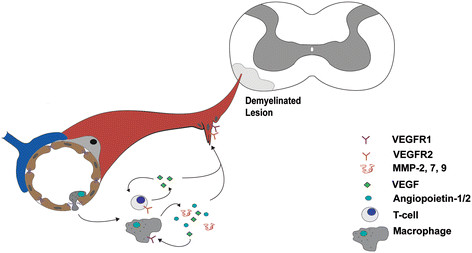
Figure 1
Figure 1 caption
Inflammatory cell infiltration is linked to angiogenesis in both MS and EAE. Inflammatory cells (T cells and macrophages) infiltrate the central nervous system parenchyma of a demyelinated lesion in both human MS or mouse EAE (depicted in the schematic diagram). After entering the hypoxic environment of the lesion, T cells and macrophages secrete pro-angiogenic factors (VEGF, angiopoietin1/2, and MMP-2, −7, −9) that both promote angiogenesis and exacerbate lesion pathology. These factors act both in a paracrine manner in endothelial cells to stimulate angiogenesis, as well as in an autocrine fashion, to exacerbate the inflammatory response of both T cells and macrophages.
Macrophages make up a large component of the inflammatory infiltrate in MS and EAE [35]. The phenotype of macrophage subtypes that develop during EAE is largely influenced by both exposure to T cells that are present in the lesion and the local hypoxic/inflammatory environment. INFγ, the predominant cytokine of inflammatory Th1 cells, shifts macrophages into a pro-inflammatory M1 phenotype [36]. M1 macrophages are predominantly found in active inflammatory MS lesions and the early stages of EAE in rats [37],[38]. However, the fraction of macrophages that exhibit M2 markers is increased at both the peak stage of the disease as well as during the remission phase, at least in the relapsing-remitting EAE model in rats [37],[38]. The complexity in phenotype switching that occurs in macrophages subtypes during MS lesion development and EAE disease underscores the complex and dynamic environment that these cells are exposed to as they cross the damaged BBB during disease progression [39]. Anti-inflammatory M2 macrophages, and not type M1, have been shown to have angiogenesis-promoting properties
In addition to contributing to ECM modifications and facilitating angiogenesis, macrophages also produce several angiogenic factors including VEGF, platelet-derived growth factor (PDGF) and angiopoietin-1 and - 2 [47] (Figure 1). The angiogenic contribution of macrophages is not limited to direct secretion, however, because they also play a role in freeing the reservoir of VEGF that is bound to the ECM by secreting MMPs that degrade the matrix and liberate bound VEGF [48]. Macrophages also express VEGFR-1, and can respond in a paracrine fashion to VEGF that they either produce or liberate from the ECM, by migrating towards angiogenic environments [47]. Consequently, the role played by infiltrating macrophages in MS and EAE is multifaceted, with overlapping functions contributing to both angiogenic and inflammatory responses (Figure 1).
Several compelling studies have investigated the effects of angiogenesis blockade on EAE progression and inflammation. Bevacizumab, a monoclonal antibody that binds VEGF and prevents interaction with its receptor [49], significantly decreases both EAE clinical scores and the number of CD4+ T cells in the CNS [27] when administered to mice on the day they began to show clinical symptoms. Similar results have been observed using the VEGFR2 receptor inhibitor SU5416, with mice showing significantly reduced acute EAE clinical scores and inflammatory infiltration, as well as fewer blood vessels per tissue section, when compared to untreated EAE mice [24]. Use of a second monoclonal VEGF-binding antibody, B20-4.1.1 [50], has recently shed light on the mechanism by which inhibiting angiogenesis may ameliorate EAE. B20-4.1.1 significantly reduced angiogenesis in the CNS, vascular permeability and clinical EAE scores during disease progression, without the concomitant reduction in CNS T cell infiltration seen using Bevacizumab or SU5416 [51]. Nevertheless, B20-4.1.1 diminished peripheral T cell activation, which was attributed by the authors to their observed reduction in EAE scores. Therefore, this study would suggest that CNS angiogenesis is linked to peripheral T cell activation rather than CNS accumulation, and provides a more complex perspective on possible secondary effects that inhibition of VEGF signaling has for CNS vasculature.
Whereas indirect inhibition of angiogenesis, by blocking angiogenic factors such as VEGF, shows promise for attenuating disease progression in EAE [24],[27],[51], direct inhibition also has clinical benefits in the EAE animal model [51]. Direct inhibition of angiogenesis by administering K(1–3) [50], a compound that contains the first 3 kringle domains from angiostatin and acts directly on vascular endothelial cells to inhibit angiogenesis [52], reduces both EAE clinical scores and IL-17 production by peripheral T cells without diminishing CNS infiltration [51]. While the precise mechanism of action underlying this phenomenon is unknown, it is clear that altering levels of angiogenesis is a potentially promising therapeutic approach for treating MS.
Potential signals regulating angiogenesis
Since a hypoxia-like state is an important component of multiple sclerosis pathology [30],[31], factors that function as angiogenic signals in either ischemic diseases (e.g. stroke) or tumor-induced angiogenesis may also promote angiogenesis in both MS and EAE [53],[54]. High levels of hypoxia-inducible factor 1 (HIF-1), a transcription factor essential for VEGF-induced angiogenesis, have been found in active MS lesions [26],[30],[55],[56]. Similar expression patterns for VEGF have been reported, with many VEGF-expressing cells observed in or adjacent to active lesions for both MS and EAE [17],[22]. Furthermore, endothelin- 1 [57] and angiopoietin-2 [27] have both been shown to enhance the angiogenic effects of VEGF, and are significantly elevated in serum from MS patients [27],[58]. The abundant evidence for both VEGF and VEGF-enhancing factor expression demonstrates a key role for this pathway in CNS angiogenesis during MS progression. NO is a ubiquitous molecule with numerous functions, including vasodilation and neurotransmission. It has been shown that NO contributes both directly and indirectly to neo-angiogenesis in inflammatory diseases [59]. Giovannoni
In addition to hypoxia-associated factors, many molecules that play a role in the pathology of MS also have angiogenesis-modulating characteristics. Tumor necrosis factor-alpha (TNFα) and INFγ, two prominent inflammatory cytokines that are present in EAE and MS [64], can promote angiogenesis [65]. Moreover, some MMPs (−1, −2 and −9) promote invasion of inflammatory cells into the CNS during MS progression [66], and contribute to proteolytic degradation of the extracellular matrix to allow angiogenic sprouting [67]. Further studies are needed to evaluate any
Conclusions
Much progress has been made in determining the contribution that angiogenesis makes during MS disease progression. Using the EAE animal model and human MS tissue samples, it is increasingly clear that neo-angiogenesis is stimulated during disease progression [15],[17],[18],[22]-[24],[27]. Furthermore, pharmacological inhibition of angiogenesis with various compounds suggests that it is beneficial for disease outcome [24],[27],[51]. Continued investigation into the potential signals that modulate the angiogenic response has already yielded therapeutic targets for treatment, and may provide additional candidates. While the mechanisms used by current anti-angiogenic compounds have yet to be elucidated, their efficacy supports ongoing research into their potential as therapeutic agents.
Acknowledgments
JL, TC and DA are supported by funding from the National Heart, Lung and Blood Institute of the National Institutes of Health (#1 R01 HL116995-01) and the National Multiple Sclerosis Society (#RG 4673A1/1).
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
References
- Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907-911.
- Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1810-1819.
- Experimental autoimmune encephalomyelitis: the antigen specificity of T lymphocytes determines the topography of lesions in the central and peripheral nervous system. Lab Investig. 1997;76:355-364.
- Physiological roles of matrix metalloproteinases: implications for tumor growth and metastasis. Can J Physiol Pharmacol. 1999;77:465-480.
- Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37:13-25.
- The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178-201.
- Development of the blood–brain barrier. Cell Tissue Res. 2003;314:119-129.
- Pericytes regulate the blood–brain barrier. Nature. 2010;468:557-561.
- Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci. 2009;106:641-646.
- Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature. 2010;468:562-566.
- Wnt/beta-catenin signaling controls development of the blood–brain barrier. J Cell Biol. 2008;183:409-417.
- Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247-1250.
- Chen J, Venkat P, Zacharek A, Chopp M: Neurorestorative therapy for stroke. Front Hum Neurosci 2014, 8:382.,
- Girolamo F, Coppola C, Ribatti D, Trojano M: Angiogenesis in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathologica Commun 2014, 2:84.,
- Ludwin SK: Vascular proliferation and angiogenesis in multiple sclerosis: Clinical and pathogenetic implications. J Neuropathol Exp Neurol 2001, 60:505.,
- . Pathological Histology: An Introduction to the Study of Pathological Anatomy. 1872.
- Vascular endothelial growth factor is expressed in multiple sclerosis plaques and can induce inflammatory lesions in experimental allergic encephalomyelitis rats. J Neuropathol Exp Neurol. 2002;61:914-925.
- Increased blood vessel density and endothelial cell proliferation in multiple sclerosis cerebral white matter. Neurosci Lett. 2010;470:65-70.
- MR-derived cerebral blood volume maps: issues regarding histological validation and assessment of tumor angiogenesis. Magn Reson Med. 2001;46:735-747.
- Changes in cerebral perfusion precede plaque formation in multiple sclerosis: a longitudinal perfusion MRI study. Brain. 2004;127:111-119.
- White matter and deep gray matter hemodynamic changes in multiple sclerosis patients with clinically isolated syndrome. Magn Reson Med. 2012;68:1932-1942.
- Seabrook TJ, Littlewood-Evans A, Brinkmann V, Pöllinger B, Schnell C, Hiestand PC: Angiogenesis is present in experimental autoimmune encephalomyelitis and pro-angiogenic factors are increased in multiple sclerosis lesions. J Neuroinflammation 2010, 7:95.,
- VEGF and vascular changes in chronic neuroinflammation. J Autoimmun. 2003;21:353-363.
- VEGF and angiogenesis in acute and chronic MOG((35–55)) peptide induced EAE. J Neuroimmunol. 2009;209:6-15.
- Extensive vascular remodeling in the spinal cord of pre-symptomatic experimental autoimmune encephalomyelitis mice; increased vessel expression of fibronectin and the alpha5beta1 integrin. Exp Neurol. 2013;250:43-51.
- Angiogenesis in multiple sclerosis: is it good, bad or an epiphenomenon?. J Neurol Sci. 2004;217:125-130.
- Bevacizumab diminishes experimental autoimmune encephalomyelitis by inhibiting spinal cord angiogenesis and reducing peripheral T-cell responses. J Neuropathol Exp Neurol. 2012;71:983-999.
- Chronic mild hypoxia ameliorates chronic inflammatory activity in myelin oligodendrocyte glycoprotein (MOG) peptide induced experimental autoimmune encephalomyelitis (EAE). Adv Exp Med Biol. 2011;701:165-173.
- Angiogenesis induced by CNS inflammation promotes neuronal remodeling through vessel-derived prostacyclin. Nat Med. 2012;18:1658-1664.
- Hypoxia-like tissue injury as a component of multiple sclerosis lesions. J Neurol Sci. 2003;206:187-191.
- Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8:280-291.
- Induction of nitric oxide synthase in demyelinating regions of multiple sclerosis brains. Ann Neurol. 1994;36:778-786.
- Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc. 2007;2:2295-2301.
- Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol. 2004;172:4618-4623.
- Macrophages: A double-edged sword in experimental autoimmune encephalomyelitis. Immunol Lett. 2014;160:17-22.
- Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17:109-118.
- Mechanism of experimental autoimmune encephalomyelitis in Lewis rats: recent insights from macrophages. Anatomy Cell Biol. 2012;45:141-148.
- Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult Scler. 2011;17:2-15.
- Vogel DY, Vereyken EJ, Glim JE, Heijnen PD, Moeton M, van der Valk P, Amor S, Teunissen CE, van Horssen J, Dijkstra CD: Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation 2013, 10:35.,
- Buhler LA, Samara R, Guzman E, Wilson CL, Krizanac-Bengez L, Janigro D, Ethell DW: Matrix metalloproteinase-7 facilitates immune access to the CNS in experimental autoimmune encephalomyelitis. BMC Neurosci 2009, 10:17.,
- Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J Neuropathol Exp Neurol. 1996;55:300-309.
- The expression profile of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in lesions and normal appearing white matter of multiple sclerosis. Brain. 2001;124:1743-1753.
- Matrix metalloproteinase-9 and −7 are regulated in experimental autoimmune encephalomyelitis. Brain. 1998;121(Pt 1):159-166.
- New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161-174.
- Gelatinase B modulates selective opening of the blood–brain barrier during inflammation. Am J Physiol. 1998;274:R1203-R1211.
- Human endothelial gelatinases and angiogenesis. Int J Biochem Cell Biol. 2001;33:960-970.
- Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev. 2008;27:31-40.
- Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681-691.
- Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593-4599.
- Antiangiogenic cancer therapy. Semin Cancer Biol. 2004;14:139-145.
- MacMillan CJ, Doucette CD, Warford J, Furlong SJ, Hoskin DW, Easton AS: Murine experimental autoimmune encephalomyelitis is diminished by treatment with the angiogenesis inhibitors B20-4.1.1 and angiostatin (K1-3). PLoS One 2014, 9:e89770.,
- Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci. 1999;96:2811-2816.
- Cell type specific upregulation of vascular endothelial growth factor in an MCA-occlusion model of cerebral infarct. J Neuropathol Exp Neurol. 1999;58:654-666.
- Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. J Neuro-Oncol. 2000;50:121-137.
- A new paraclinical CSF marker for hypoxia-like tissue damage in multiple sclerosis lesions. Brain. 2003;126:1347-1357.
- IL-1beta regulates blood–brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol. 2006;177:5574-5584.
- Insulin-like growth factor I stimulates angiogenesis and the production of vascular endothelial growth factor. Growth Hormon IGF Res. 2000;10(Suppl A):S41-S42.
- Increased endothelin-1 plasma levels in patients with multiple sclerosis. J Neuroophthalmol. 2001;21:37-38.
- Nitric oxide and angiogenesis. J Neuro-Oncol. 2000;50:139-148.
- Serum inflammatory markers and clinical/MRI markers of disease progression in multiple sclerosis. J Neurol. 2001;248:487-495.
- Hypoxia and angiogenesis in rheumatic diseases. Z Rheumatol. 2003;62:II43-II45.
- Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500-508.
- Upregulation of vascular growth factors in multiple sclerosis: correlation with MRI findings. J Neurol Sci. 2006;243:21-30.
- Ulinastatin attenuates experimental autoimmune encephalomyelitis by enhancing anti-inflammatory responses. Neurochem Int. 2014;64:64-72.
- Tumour necrosis factor and cancer. J Pathol. 2013;230:241-248.
- Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med. 1997;75:165-173.
- Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267-285.

