Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury
Vascular Cell. 2014;
Received: 11 August 2014 | Accepted: 10 September 2014 | Published: 1 October 2014
Vascular Cell ISSN: 2045-824X
Abstract
Background
The origins of neointimal smooth muscle cells that arise following vascular injury remains controversial. Studies have suggested that these cells may arise from previously differentiated medial vascular smooth muscle cells, resident stem cells or blood born progenitors. In the current study we examined the contribution of the previously differentiated vascular smooth muscle cells to the neointima that forms following carotid artery ligation.
Methods
We utilized transgenic mice harboring a cre recombinase-dependent reporter gene (mTmG). These mice express membrane targeted tandem dimer Tomato (mTomato) prior to cre-mediated excision and membrane targeted EGFP (mEGFP) following excision. The mTmG mice were crossed with transgenic mice expressing either smooth muscle myosin heavy chain (
Results
Analysis of the cellular composition of the neointima that forms following injury revealed that mEGFP positive cells derived from either
Conclusion
These data demonstrate that the majority of the neointima that forms following carotid ligation is derived from previously differentiated medial vascular smooth muscle cells.
Keywords
Vascular smooth muscle Neointima Smooth muscle myosin Smooth muscle α-actinBackground
Vascular smooth muscle cells (VSMCs) are the major contractile components of the vascular system. They are critically important for regulating blood pressure and flow throughout the vascular system. Unlike skeletal and cardiac muscle cells, VSMCs are remarkably plastic and modulate their phenotype in response to extracellular cues during the development and progression of a variety of diseases including atherosclerosis, hypertension, stenosis following injury and restenosis following vascular interventions. Cardiovascular disease is the leading cause of death among the US population, yet despite intense research efforts a number of basic questions regarding the etiology of cardiovascular disease remain elusive. Classically these diseases are described as being associated with dedifferentiated VSMCs that have decreased expression of proteins required for the normal contractile function, increased expression of extracellular matrix proteins and increased cell proliferation [1]. These proliferating dedifferentiated VSMCs are a major component of neointimal lesions and atherosclerotic plaques. Neointimal VSMCs have been proposed to arise from several sources, including blood and bone marrow derived precursor cells, dedifferentiated medial VSMCs, resident progenitor cells and adventitial fibroblasts. However, recent definitive studies showed a relatively minor contribution of blood and bone marrow derived cells to the neointima or atherosclerotic plaque VSMC population [2–5]. The most widely accepted paradigm that neointimal VSMCs arise from the dedifferentiation and migration of medial VSMCs has been recently challenged [6]. This finding has stimulated much controversy in the field [7] and has prompted us to further investigate the origin of these cells. Using a genetic fate mapping approach with tamoxifen regulated smooth muscle-specific cre recombinase and a dual color cre-dependent reporter gene we unequivocally show that the neointimal SMCs that arise following carotid artery ligation are largely derived from the previously differentiated medial VSMCs.
Methods
Transgenic mice and carotid ligation
All animal procedures were performed using procedures approved by the Indiana University School of Medicine Institutional Animal Care and Use committee under protocol number 10310. Smooth muscle myosin heavy chain (
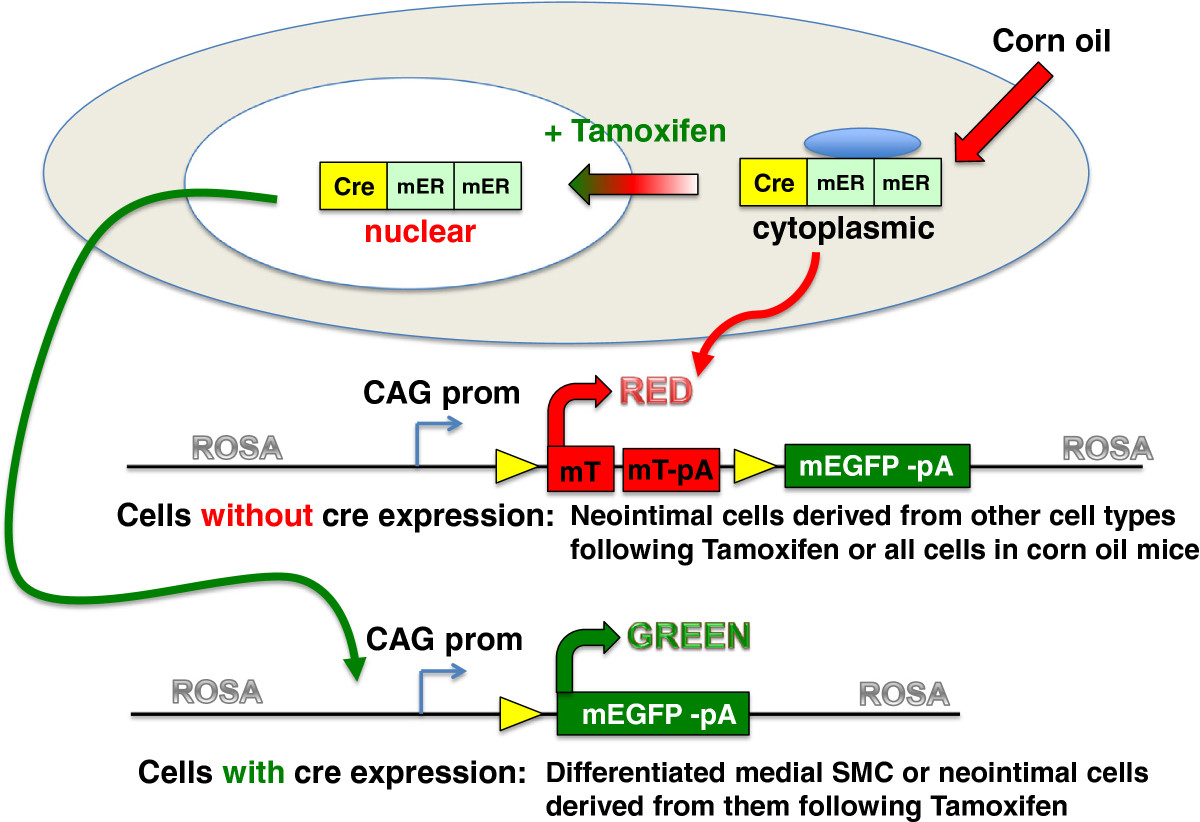
Figure 1
Figure 1 caption
Schematic representation of the mTmG reporter strain. The mTmG reporter mice (Jackson strain: B6.129 (Cg)-Gt(ROSA)26Sor/J ) contain a single copy of the transgene integrated into the ROSA 26 locus. The transgene cassette is comprised of a chimeric CMV, β-actin promoter driving the expression of a floxed membrane localized Tomato tandem dimer. Following cre-mediated excision the mTomato transgene is removed such that the CAG promoter now drives expression of membrane localized EGFP. The reporter mice were crossed with transgenic mice that express a tamoxifen regulated cre recombinase directed to smooth muscle cells by either the promoter or the promoter. Double heterozygous transgenic mice were used for all experiments.
Histological and immuno-staining
OCT was removed from sections by 3, 5 minute washes in Tris buffered saline (TBS; 100 mM Tris pH7.6, 150 mM NaCl). Slides were mounted in Prolong Gold containing DAPI (Invitrogen) and visualized by confocal microscopy (Olympus Fluoview FV1000). For immuno-fluorescent staining cryosections were permeabilized in 0.2% triton in TBS for 5 minutes, washed in TBS for 5 minutes them blocked in 5% goat serum diluted in TBS at room temperature for 1 hour. Blocked sections were incubated for 4-5 hours at 37° with primary antibodies to, the SM2 isoform of smooth muscle myosin heavy chain (1:500) [11], CD31 (1:100, clone 390, Affymetrix, eBioscience), CD68 (1:200, clone FA-11, AbD Serotec) diluted in 5% goat serum/TBS. Some sections were incubated without primary antibody as a negative control. Following washing in TBS primary antibodies were detected by incubation with anti-rabbit or anti-rat Alexa Fluor 647 (1:10,000, Jackson ImmunoResearch). After washing slides were mounted in Prolong Gold containing DAPI (Invitrogen) and visualized by confocal microscopy.
Quantitation of mEGFP and mTomato positive cells in lesions
In order to count the number of mEGFP and mTomato positive cells within the neointima of each vessel, a Z-stack series of 1 μm optical sections were obtained from each vessel. mEGFP and mTomato positive cells were counted in each of the optical sections through the entire Z-stack of images, each DAPI positive nuclei was scored as being associated with either an mEGFP or mTomato positive cell. For some sections it was possible to obtain 2 different fields of Z-stack images.
Results
Results of a recent study suggest that neointimal cells that form following vascular injury are derived from a stem cell population resident within the vascular wall rather than from previously differentiated VSMCs [6]. In that study fate mapping experiments were performed in which differentiated VSMCs were tagged using a cre-dependent EGFP reporter strain crossed with transgenic mice expressing cre recombinase exclusively in smooth muscle cells (B6.Cg-Tg(Myh11-cre,-EGFP)2Mik/J) [12]. The conclusion that previously differentiated VSMCs do not contribute to neointima formation was thus, based largely on the inability to detect EGFP-positive cells in the neointima in these mice. As there are many factors that can contribute to a negative result in these experiments, including the reported silencing of the ROSA 26 promoter that was used to drive EGFP expression in neointimal cells [13], we reevaluated these findings using an alternative fate mapping strategy. We utilized a dual color reporter transgenic mouse line (mTmG: B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J) in which all cells express a membrane localized mTomato tandem dimer in the absence of cre recombinase activity. Following cre-mediated excision of the mTomato cassette, cells express membrane localized EGFP [14] (Figure 1). In this reporter mouse line, both mTomato and mEGFP are driven by the same CMV/β-actin (CAG) promoter that has been shown to be active in neointimal cells [13]. This dual color reporter strain obviates the need to interpret negative data, as all neointimal cells should be either red or green depending on whether they express mTomato of mEGFP, respectively. To specifically identify differentiated VSMCs we crossed the mTmG mice with mice expressing a tamoxifen regulated cre recombinase directed by the smooth muscle-specific
In
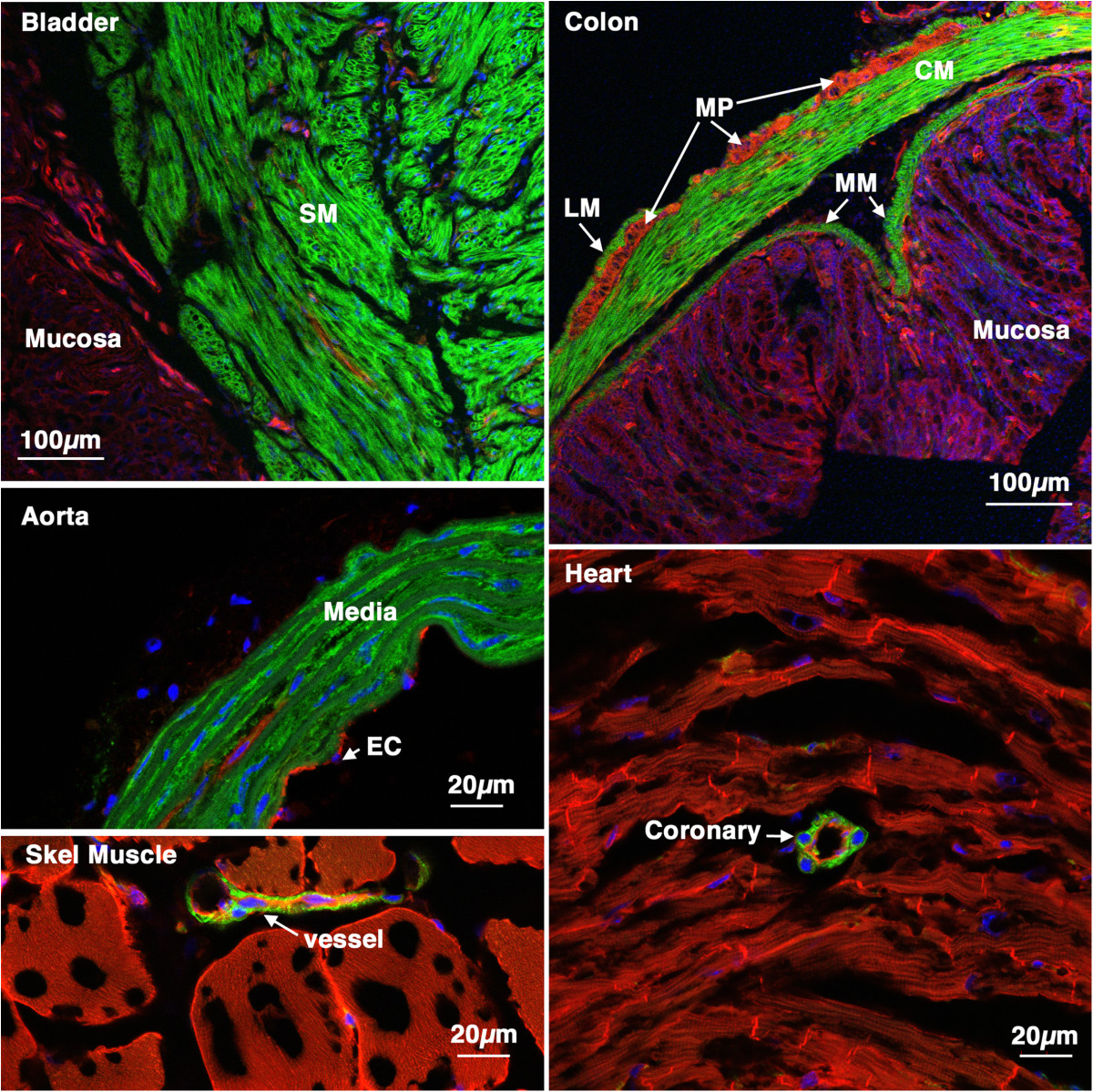
Figure 2
Figure 2 caption
Tissue specificity ofcre ER(T2) activity. 5-week old creER(T2) mTmG mice were treated with tamoxifen (1 mg,IP) once a day for 5 days. 6 weeks later tissues were harvested and analyzed by confocal microscopy as described in ‘Methods’. A strong mEGFP signal can be seen only in smooth muscle cells of all the tissues examined including, bladder, colon, aorta, skeletal muscle and heart. No cre activity was detected in other cell types including skeletal muscle cells cardiac myocytes, endothelial cells or mucosal epithelial cells. SM-smooth muscle, LM-longitudinal muscle, CM-circular muscle, MP-myenteric plexus, MM-muscularis mucosa, EC-endothelial cells.
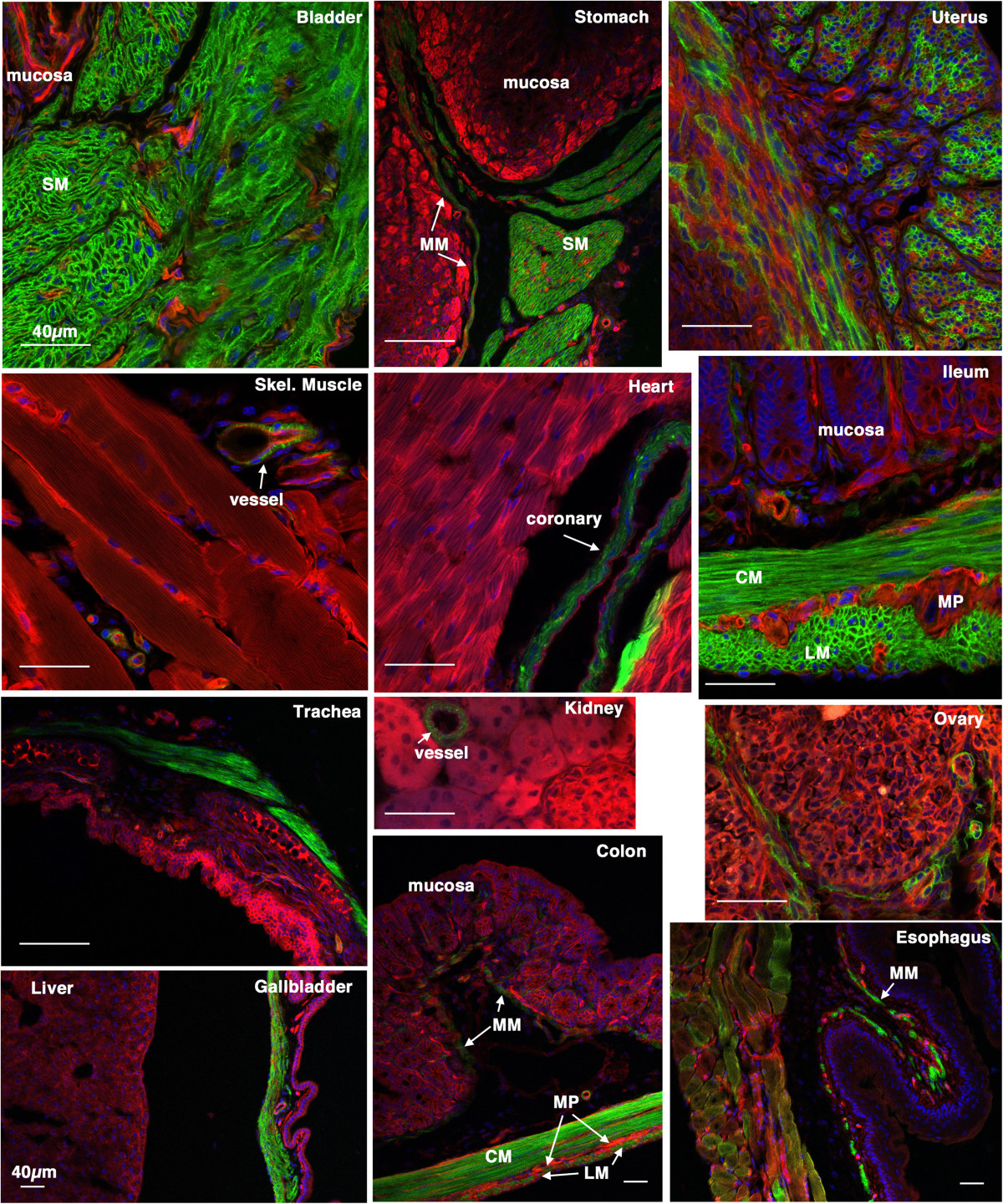
Figure 3
Figure 3 caption
Tissue specificity ofcre ER(T2) activity. 5-week old creER(T2) mTmG mice were treated with tamoxifen (1 mg,IP) once a day for 5 days. 2 weeks following the last tamoxifen injection the left carotid artery was ligated. 4 weeks later tissues were harvested and analyzed by confocal microscopy as described in ‘Methods’. A strong mEGFP signal can be seen only in smooth muscle cells of all the tissues examined. No cre activity was detected in other cell types including skeletal muscle cells, cardiac myocytes, endothelial cells, mucosal epithelial cells and hepatocytes. A heterogeneous staining was observed in the uterus suggesting that cre was not active in all the uterine smooth muscle cells during the period in which the mice were treated with tamoxifen. The patchwork mEGFP expression observed in the esophagus reflects the mixed skeletal/smooth muscle lineage of cells within the wall of this portion of the esophagus. SM-smooth muscle, LM-longitudinal muscle, CM-circular muscle, MP-myenteric plexus, MM-muscularis mucosa. Scale bars represent 40 μm in all panels.
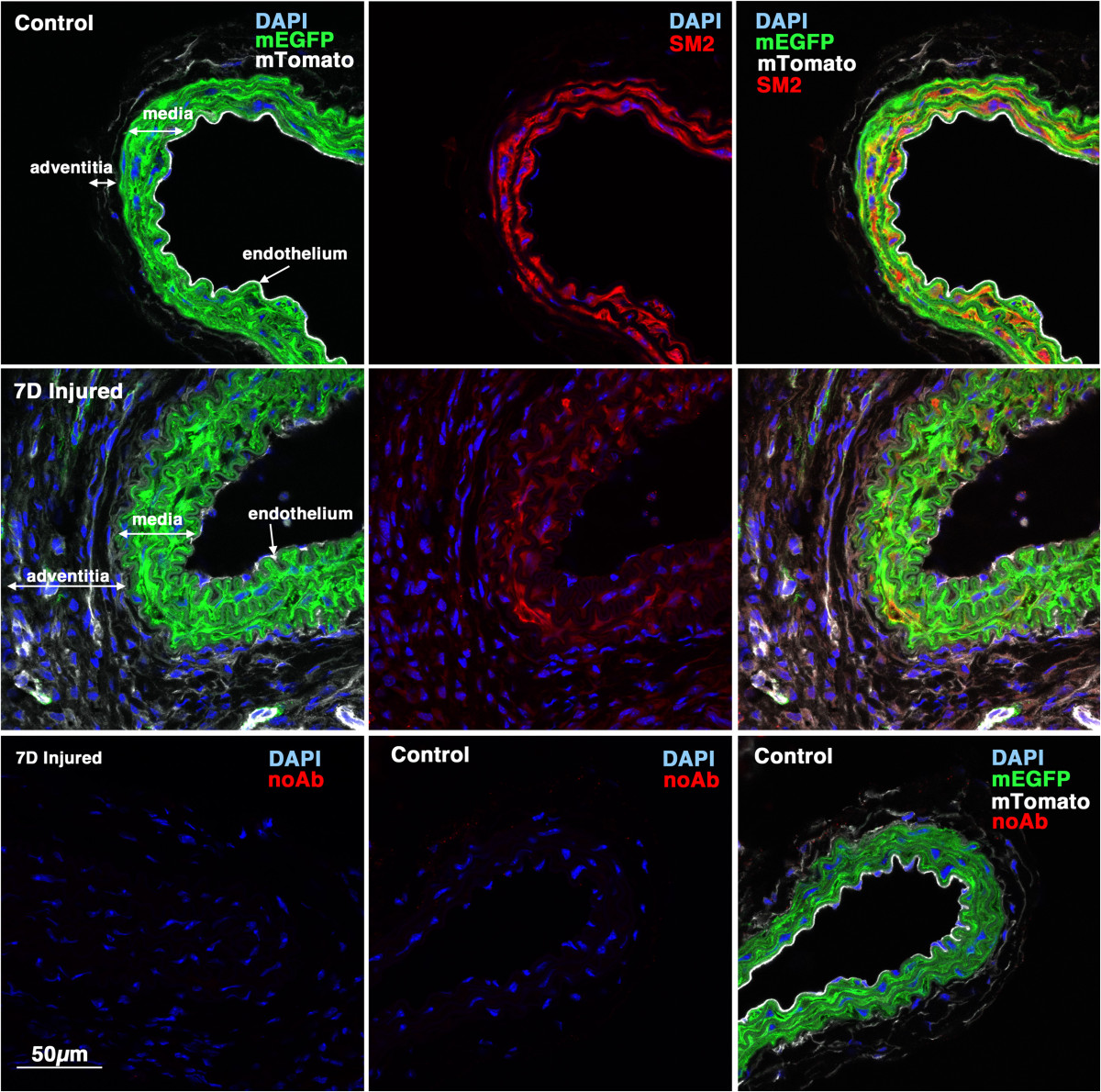
Figure 4
Figure 4 caption
Myh11 expression is down-regulated 7 days following injury. 5-week old male creER(T2) mTmG mice were treated with tamoxifen (1 mg,IP) once a day for 5 days. Two weeks following the last tamoxifen injection the left carotid artery was ligated and tissues were harvested 7 days later. 8 μm cryosections obtained from injured and contralateral control arteries were analyzed for expression of (using an anti-SM2 antibody) (red) and mEGFP (green) and mTomato (white). Control and injured sections are shown at identical exposures. Images shown are representative of those obtained from 3 different mice. In the lower panels the SM2 antibody was omitted and the samples otherwise processed identically to those shown in the upper panels. All images are shown at the same magnification with the scale bar shown in the bottom left panel representing 50 μm.
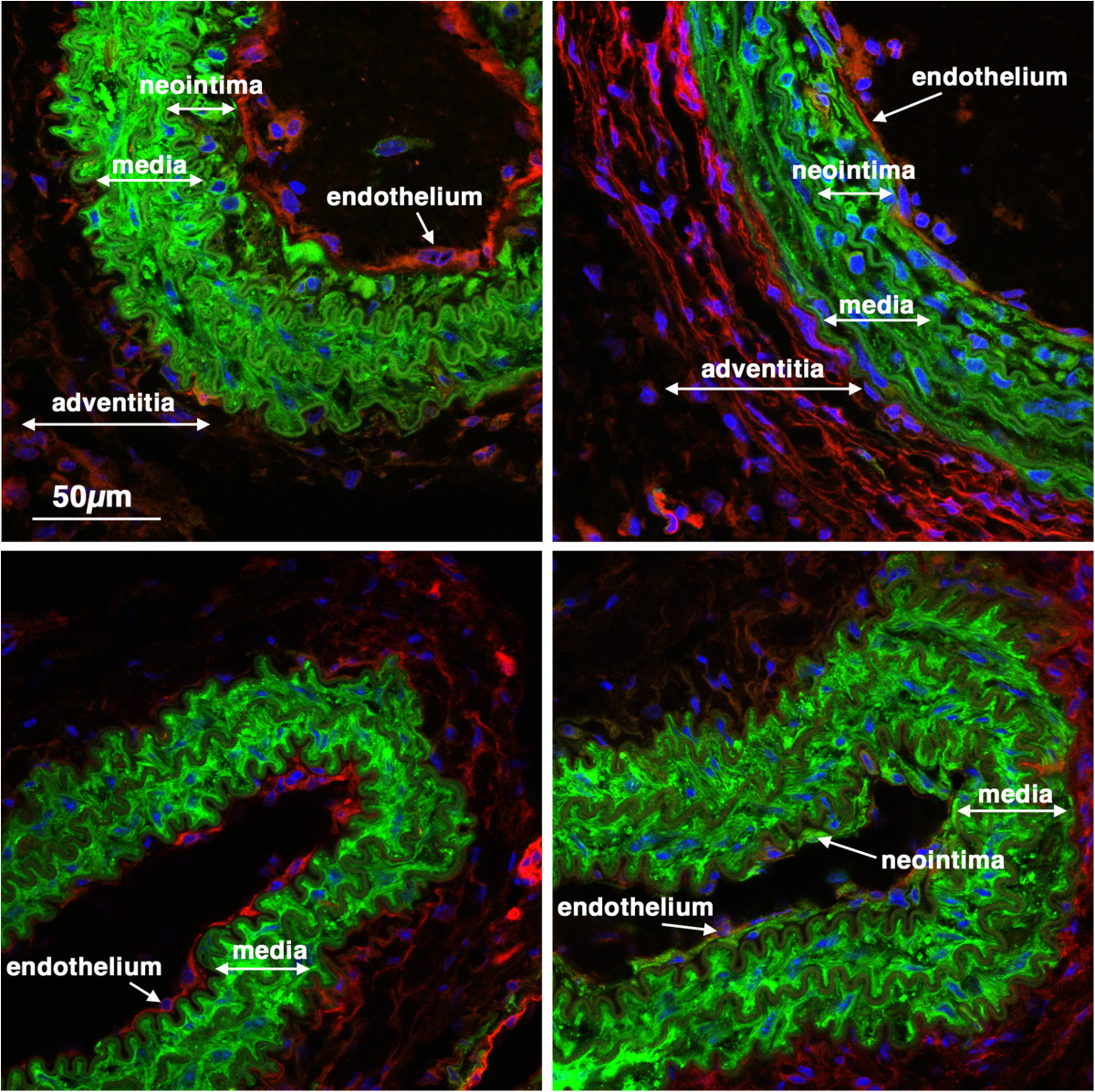
Figure 5
Figure 5 caption
Previously differentiated VSMC contribute to early neointima formation. 5-week old male creER(T2) mTmG mice were treated with tamoxifen (1 mg,IP) once a day for 5 days. Two weeks following the last tamoxifen injection the left carotid artery was ligated and tissues were harvested 14 days later. Expression of mTomato (red) and mEGFP (green) were visualized in sections obtained from 4 different mice. Nuclei were visualized by staining with DAPI (blue). All images are shown at the same magnification with the scale bar shown in the top left panel representing 50 μm.
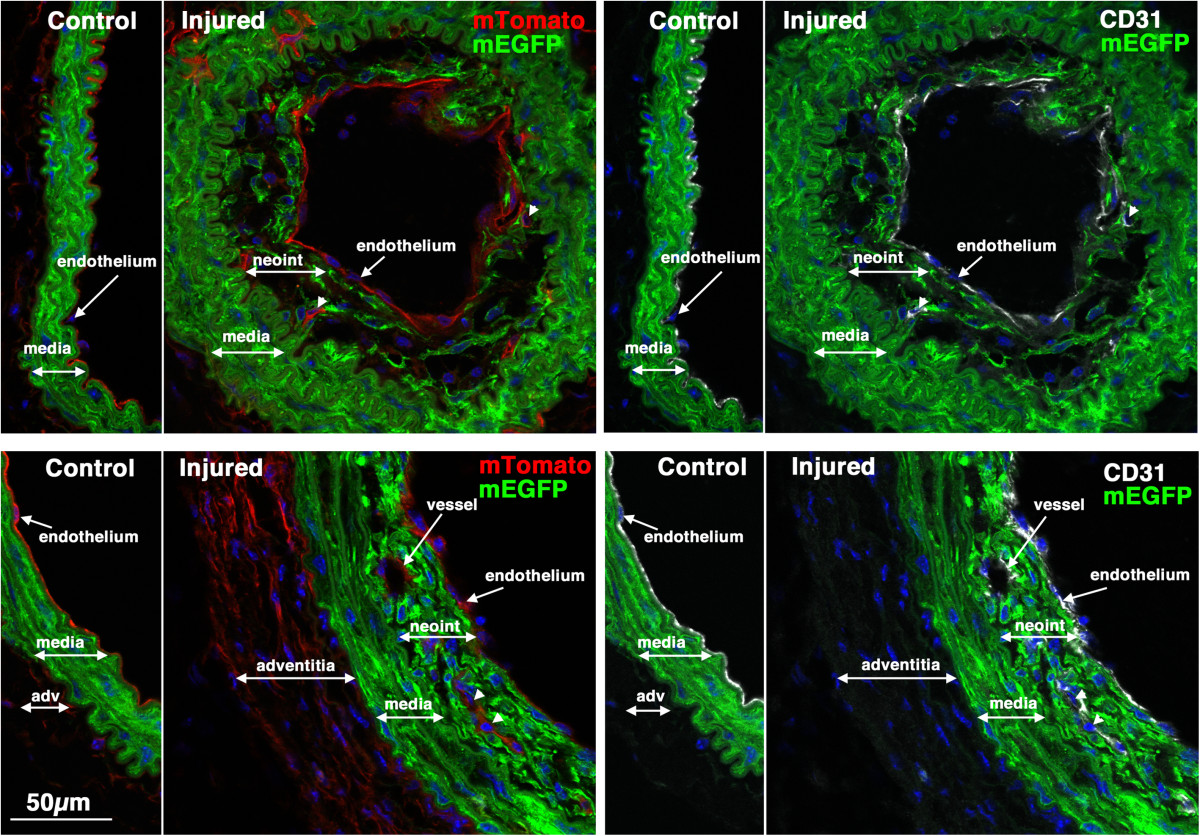
Figure 6
Figure 6 caption
CD31 positive endothelial cells can be seen within early developing neointima. Sections from two of the mice shown in Figure were stained with antibodies to CD31 (white). Left panels show mTomato (red)/mEGFP (green) co-stained sections and in the right panels are the same sections visualized for mEGFP (green) and CD31(white). Nuclei were visualized by staining with DAPI (blue). All images are shown at the same magnification with the scale bar shown in the bottom left panel representing 50 μm.
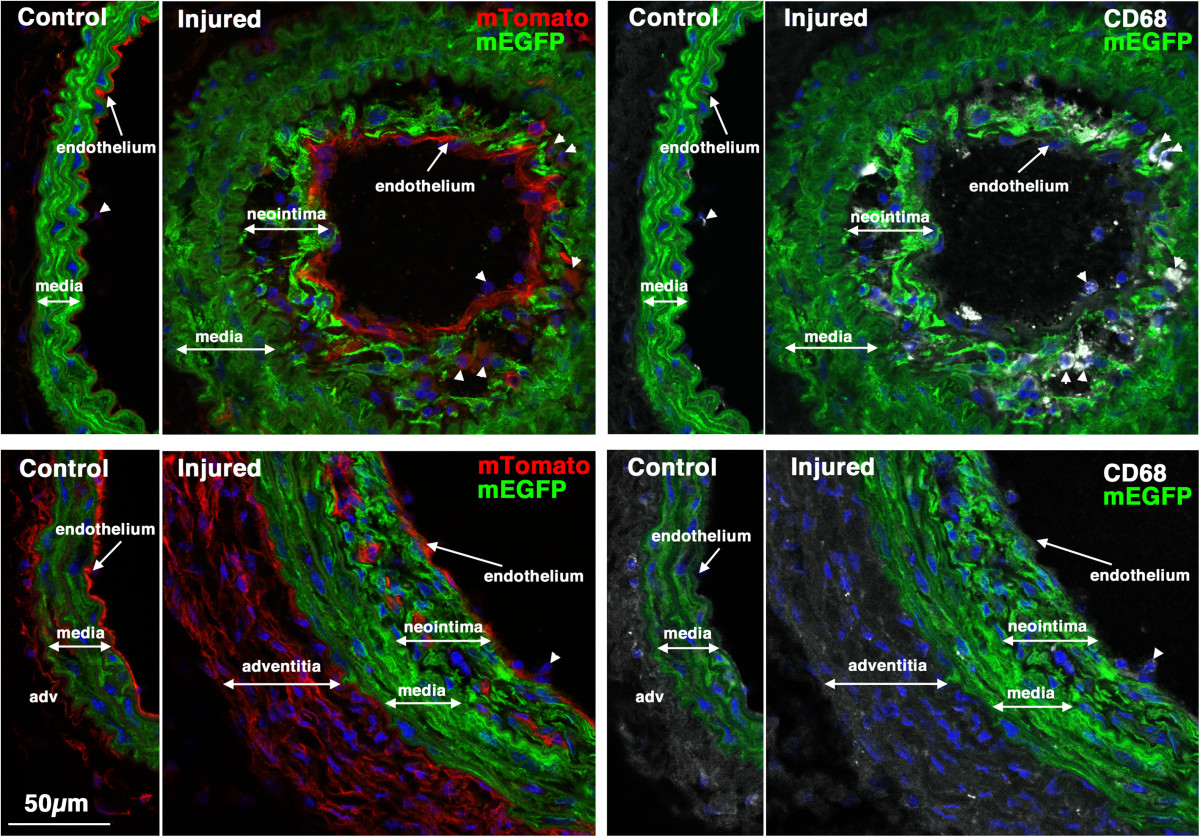
Figure 7
Figure 7 caption
CD68 positive macrophages/monocytes cells can be seen within early developing neointima in some mice. Sections from the same two mice shown in Figure were stained with antibodies to CD68 (white). Left panels show mTomato (red)/mEGFP (green) co-stained sections and in the right panels are the same sections visualized for mEGFP (green) and CD68(white). CD68-positive cells can be seen within the neointima of vessels from the mouse shown in the upper panels but not in the vessels of the mouse shown in the lower panels. Nuclei were visualized by staining with DAPI (blue). All images are shown at the same magnification with the scale bar shown in the bottom left panel representing 50 μm.
To better quantitate the contribution of mEGFP positive cells to neointima formation we examined more mature lesions that formed 28 days following ligation. In most
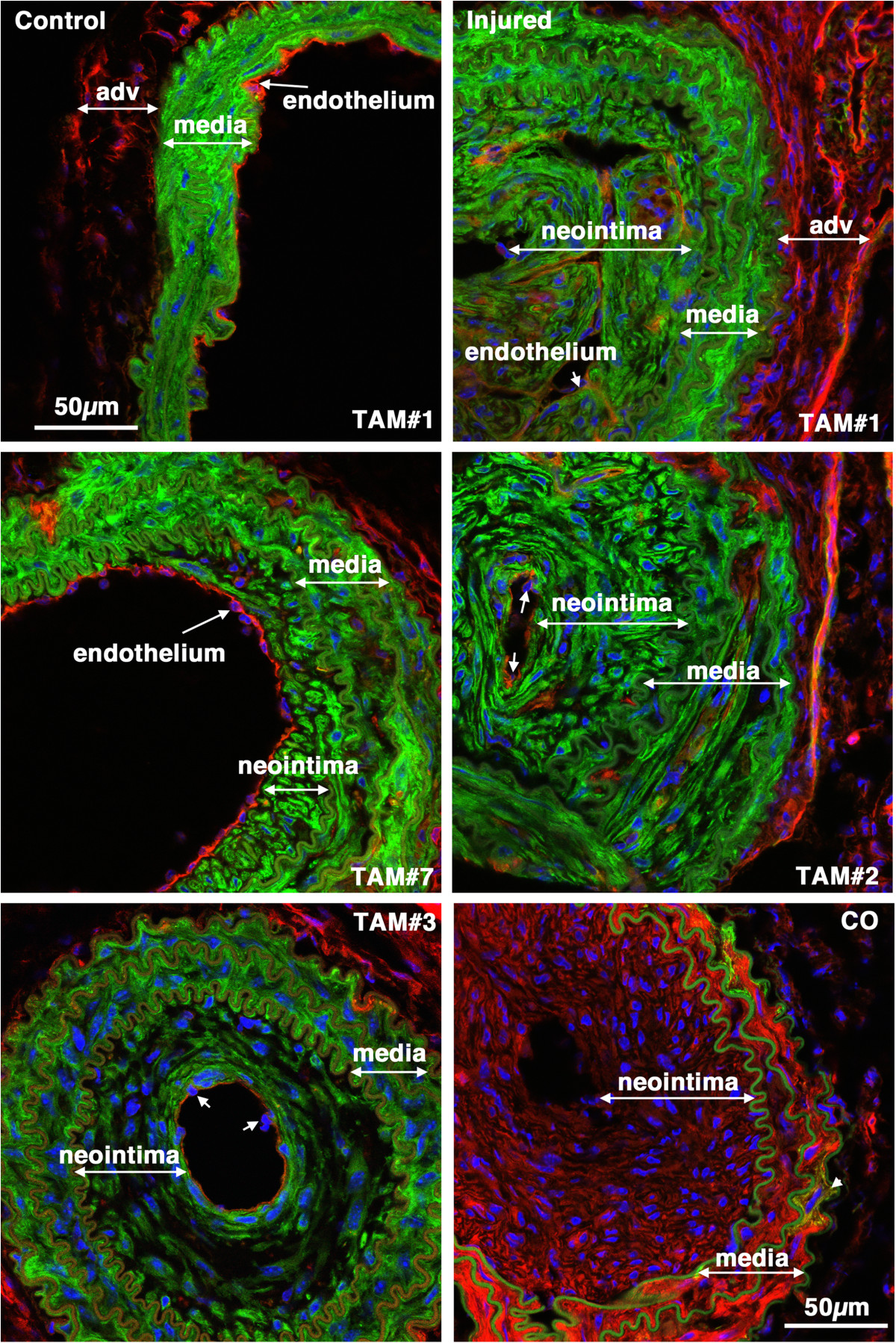
Figure 8
Figure 8 caption
-tagged medial SMCs give rise to the mature neointima following carotid ligation. Tissues were harvested 28 days following carotid ligation of 5-week old male creER(T2) mTmG mice that were previously Treated with Tamoxifen (TAM) or corn oil(CO) as indicated. In the upper two panels the control and injured carotid arteries from the same mouse are shown. The neointima, media and adventitia (adv) are indicated. Images are representative of those obtained from 7 tamoxifen treated mice (Mice numbers 1,7,2,3 in Table , as indicated). Arrows point to examples of endothelial cell nuclei. The image in the lower right hand panel is of an injured vessel obtained from a corn oil control treated mouse. The arrow-head in this image points to an mEGFP positive cell within the medial layer. All images are shown at the same magnification with the scale bars representing 50 μm.
Consistent with data obtained from the
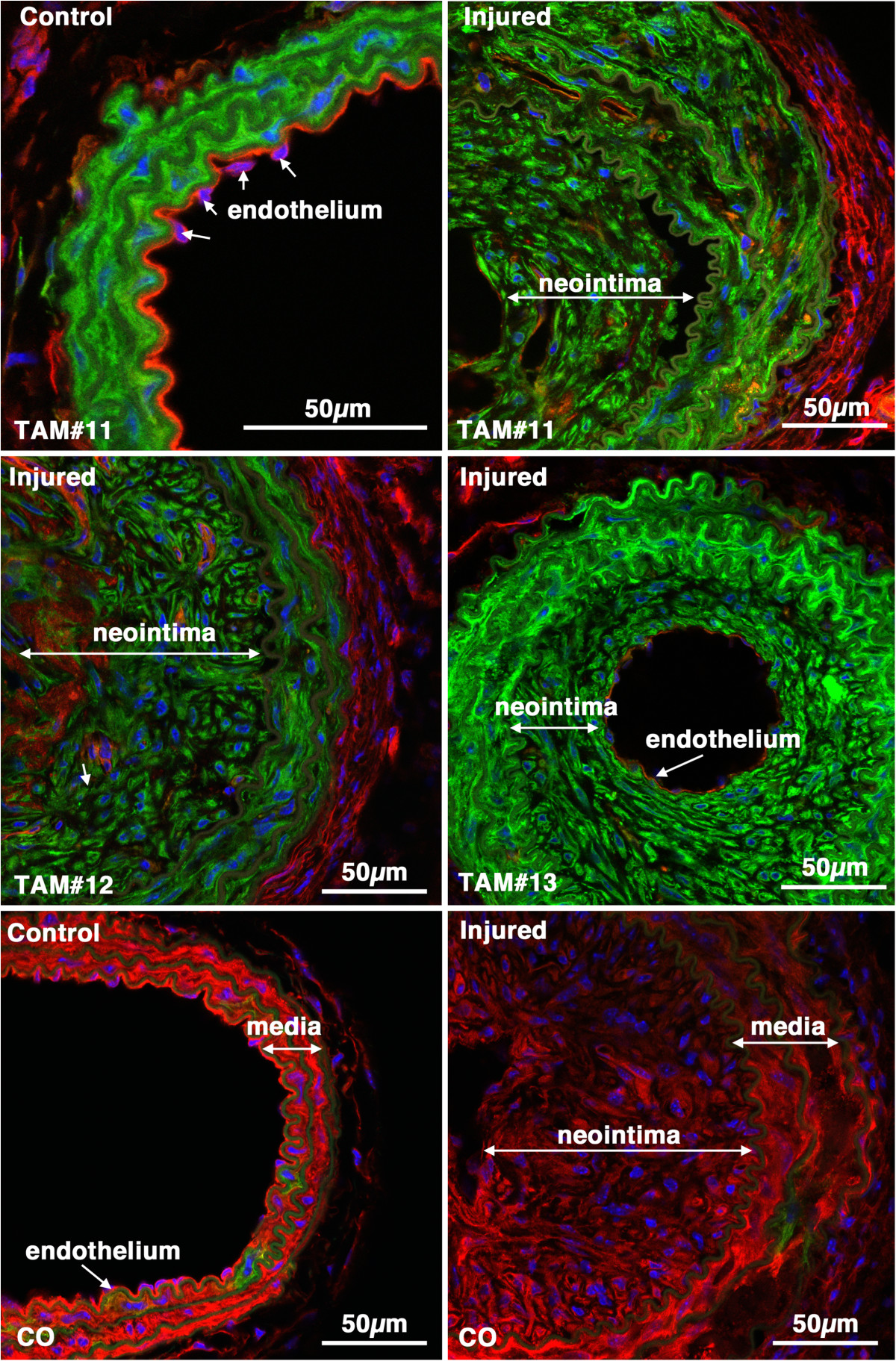
Figure 10
Figure 10 caption
-tagged medial SMCs give rise to the neointima following carotid ligation. Control and injured carotid arteries from Tamoxifen (TAM) or corn oil (CO) treated creER(T2) mTmG transgenic mice harvested 28 days post ligation (Mice numbers 11, 12 and 13, respectively; Table ). Images from tamoxifen treated injured vessels are representative of those obtained from 7 mice.
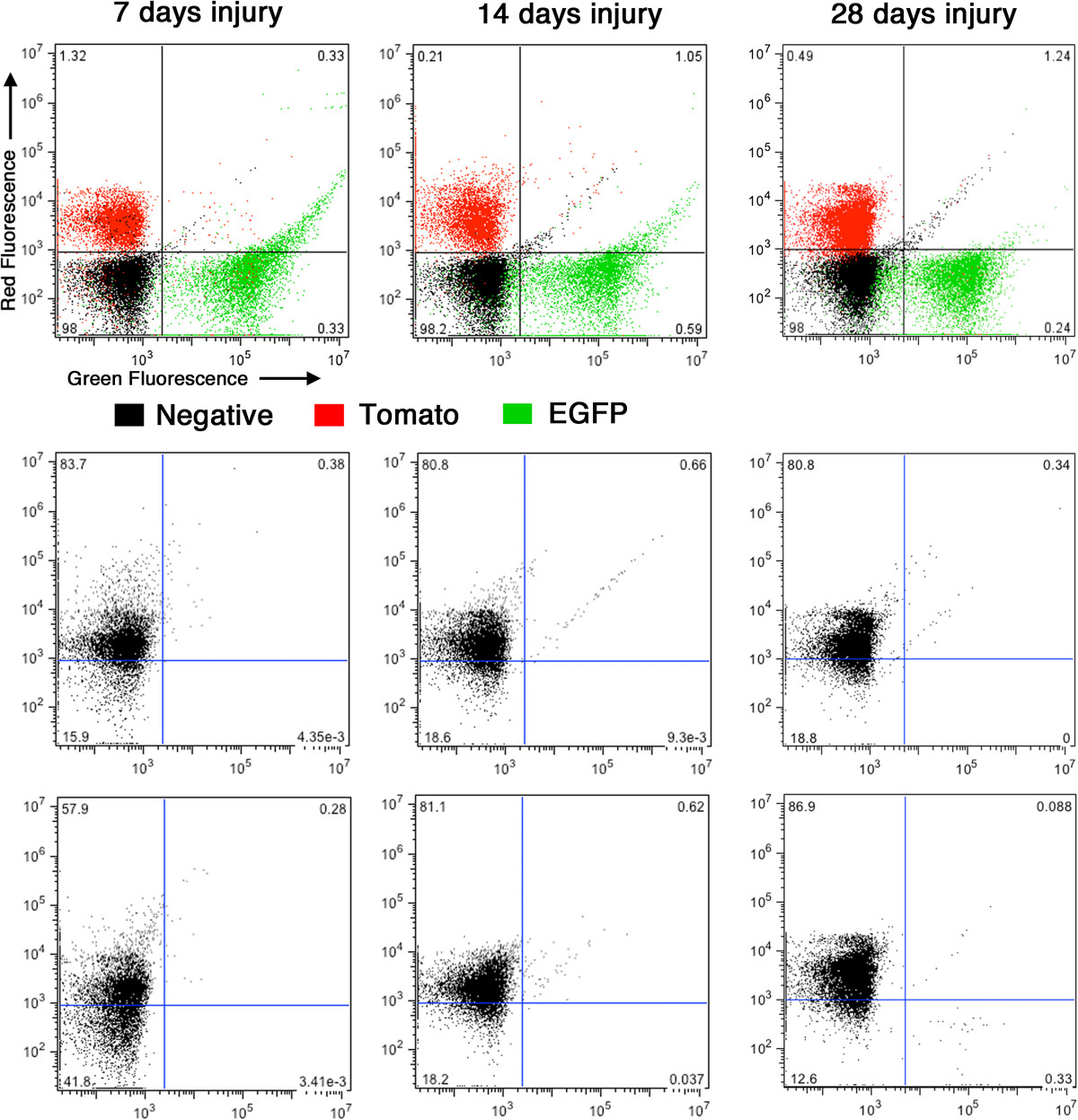
Figure 11
Figure 11 caption
No mEGFP positive circulating cells can be detected following carotid ligation. Blood was harvested from creER(T2) mTmG mice 7, 14 and 28 days following carotid ligation and subjected to fluorescence activated cell sorting. Cells harvested from a CAG-EGFP mouse were used as positive controls for EGFP expressing cells, those harvested from mTmG reporter mice with no cre transgene as positive controls for mTomato positive cells and those harvested from nontransgenic mice as negative controls. From each mouse a minimum of 10,000 cells were sorted. Upper panels show the distribution of mTomato (red) and mEGFP (green) positive as well as negative (black) cells. Numbers on each graph show the percentage of negative cells in each quadrant. Lower panels show the distribution of cells obtained from duplicate creER(T2) mTmG mice. In each case the percentage of EGFP-positive cells are less than background negative control levels.
Discussion
Results from the current studies support the widely accepted paradigm that following vascular injury medial VSMCs dedifferentiate and migrate into the lumen of vessels forming a neointima. As we utilized a tamoxifen regulated cre recombinase and waited 2 weeks after tamoxifen treatment before performing carotid ligation, given the 12 hour half life of tamoxifen in serum, it is highly unlikely that mEGFP positive cells seen following injury are derived from newly differentiated cells. Moreover, the use of a dual color reporter system avoided any artifacts that may arise due to promoter or reporter silencing as all cells should be either mTomato or mEGFP positive thus no conclusions need to be drawn that are based on negative staining data. Our data also suggest that unlike the ROSA-LACz reporter gene [13] the CMV enhancer/chicken beta-actin core promoter (CAG) driven mTmG reporter gene is not down-regulated in neointimal SMCs (compare the mEGFP intensity of control and injured vessels in Figures 3,8 and 10). The use of the mTmG reporter strain has the additional advantage that the reporter proteins are membrane localized and thus better retained during sample processing. Moreover, this is a single copy, targeted transgene, hence, it is also not subject to complications that may arise from partial recombination of multicopy transgenes, such that in each cell’s nucleus either the mTomato gene is present or it is excised. Cells will thus express either mTomato or mEGFP. Anecdotally we have noted, that the mTomato protein is relatively stable such that after tamoxifen treatment smooth muscle cells can have detectable expression of both mTomato and mEGFP for 3-4 days before the mTomato protein is turned over and degraded. We speculate that one or more of these advantages of the mTmG reporter system and tamoxifen regulated cre transgenes used in our study may explain why we were able to detect mEGFP positive neointimal cells whereas they were not detected in a previous study [6].
Our data are consistent with and extend previous fate mapping studies using a cre-dependent LacZ reporter [5]. In this study a cre-dependent ROSA-LacZ reporter was used together with
Conclusions
Although our studies do not rule out the possibility that under appropriate, in vitro, culture conditions the expansion of a progenitor cell population may be favored, the current studies provide compelling evidence that, in vivo, the majority of neointimal cells that arise following carotid ligation are derived from differentiated medial VSMCs. The lack of detectable mEGFP positive cells circulating in the blood, further suggests that the neointimal cells likely arise from the dedifferentiation and migration of locally derived medial VSMCs.
Acknowledgements
This research was supported by research support funds from IUPUI.
We would like to thank Dr. Joseph Miano, Dr. Richard Karas and Dr. Matthew Distasi for providing the
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2
Authors’ original file for figure 3
Authors’ original file for figure 4
Authors’ original file for figure 5
Authors’ original file for figure 6
Authors’ original file for figure 7
Authors’ original file for figure 8
Authors’ original file for figure 9
Authors’ original file for figure 10
Authors’ original file for figure 11
References
- Platelet-derived growth factor-BB and Ets-1 transcription factor negatively regulate transcription of multiple smooth muscle cell differentiation marker genes. Am J Physiol Heart Circ Physiol. 2004;286:H2042-H2051.
- Smooth muscle cells in transplant atherosclerotic lesions are originated from recipients, but not bone marrow progenitor cells. Circulation. 2002;106:1834-1839.
- Smooth muscle cells healing atherosclerotic plaque disruptions are of local, not blood, origin in apolipoprotein E knockout mice. Circulation. 2007;116:2053-2061.
- Bone marrow-derived cells contribute to vascular inflammation but do not differentiate into smooth muscle cell lineages. Circulation. 2010;122:2048-2057.
- SDF-1alpha induction in mature smooth muscle cells by inactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2011;31:1300-1308.
- Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012;3:875-.
- Smooth muscle cell plasticity: fact or fiction?. Circ Res. 2013;112:17-22.
- G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64-68.
- Efficient temporally-controlled targeted mutagenesis in smooth muscle cells of the adult mouse. Genesis. 2009;47:14-18.
- Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238-2244.
- Alterations in expression of myosin and myosin light chain kinases in response to vascular injury. Am J Physiol Cell Physiol. 2000;279:C1078-C1087.
- Smooth muscle expression of Cre recombinase and eGFP in transgenic mice. Physiol Genomics. 2002;10:211-215.
- Characterization of Pdgfrb-Cre transgenic mice reveals reduction of ROSA26 reporter activity in remodeling arteries. Genesis. 2011;49:673-680.
- A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593-605.
- Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767-801.

