External Quality Assessments of CD31 Immunoassays – the NordiQC experience
Vascular Cell. 2020;
Received: 19 May 2020 | Accepted: 29 May 2020 | Published: 30 July 2020
Vascular Cell ISSN: 2045-824X
Abstract
Nordic Immunohistochemical Quality Control (NordiQC) performs proficiency testing for about 600 pathology laboratories in more than 50 countries. All general results are published on http://www.nordiqc.org. Over-all, about 30% of the staining results on circulated slides from tissue micro arrays have been assessed as insufficient for diagnostic use by an expert group. This paper describes the results from the two latest NordiQC runs for CD31. Out of 476 stains submitted, 30.5% were considered insufficient, mostly due to too weak or false negative staining reactions. The best results were obtained by use of mouse monoclonal antibody JC70A with an optimized protocol based on efficient heat induced epitope retrieval and a three-step polymer/multimer conjugate as visualization system. The mouse monoclonal antibody 1A10 gave unsatisfactory results in almost all cases.
Keywords
CD31 Immunohistochemistry External quality assurance NordiQCIntroduction
CD31 is a transmembrane glycoprotein, about 130 kDa, also designated platelet-endothelium cell adhesion molecule (PECAM-1), belonging to the immunoglobulin super family and encoded by the PECAM-1 gene on chromosome 17 [1]. CD31 expression is restricted to endothelial cells (ECs), platelets and immune cells, suggesting that CD31 is specifically involved in the immune responses at the vascular wall [2]. Thus, during inflammation CD31 plays a major role in the adhesion cascade between endothelial cells and inflammatory cells, facilitating leucocyte migration, particularly being an important component in the regulation of neutrophil transendothelial migration [3]. CD31 also plays a role in thrombosis and angiogenesis.
Immunohistochemically, CD31 is usually strongly expressed in both blood vessel and lymphatic vessel ECs, in a distinct membranous pattern as it makes up a large portion of the intercellular junctions. In liver, the expression in ECs is lower [4] but increases with capillarization of the liver sinusoids in chronic liver diseases [5]. CD31 is varyingly expressed in myeloid progenitor cells, megakaryocytes, platelets, and neutrophil granulocytes as well as in subpopulations of B- and T-lymphocytes. Typically, mantle zone B-cells have a low expression of CD31, while plasma cells are high expressors. Among neoplasms, CD31 is expressed in the vast majority of vascular lesions, such as haemangioma, angiofibroma, and angiosarcoma, and in most cases of Kaposi sarcoma and epithelioid hemangioendothelioma [6]. In some tumours such as littoral cell angioma, identification of CD31 may be mandatory for a correct diagnosis. CD31 may also be expressed in a minority of haematolymphoid neoplasms like histiocytosis, juvenile xanthogranuloma, B- and T-cell lymphomas, plasmacytoma, and myeloid leukaemia. Among carcinomas and mesotheliomas, also a minority of cases has been reported to express CD31 [7]. CD31 assays are mostly applied in order to identify vascular ECs in various lesions (e.g., to visualize metastatic spread in lymphatic and blood vessels), assess angiogenesis (vascular density) in neoplasms and verify endothelial neoplasms. For these purposes, CD31 is often used in panels, which also may include other endothelial markers like ERG, CD34, Fli-1 and Podoplanin (D2-40)[8].
In the Nordic immunohistochemical Quality Control (NordiQC) external quality assessment (EQA) program, CD31 IHC assays have been assessed five times during 2004-2016. A large proportion of submitted assays has been assessed as insufficient (24-48%). The aim of the present paper is to give an overview of the CD31 EQA results in the two latest assessments, run 38 (2013) and run 46 (2016). Detailed assessment reports from all runs are available on the NordiQC homepage (http://www.nordiqc.org).
Materials and Methods
Tissue microarrays (TMAs) for the CD31 tests were constructed for each run comprising tissues from appendix, tonsil, liver, angiosarcoma, and (in run 46) colon adenocarcinoma. The included tissues were all fixed in 10% neutral buffered formalin for 24-48 hours. Criteria for assessing CD31 staining as optimal included:
• A strong and distinct, predominantly membranous staining reaction of virtually all normal ECs and plasma cells in appendix and tonsil
• An at least weak to moderate, distinct membranous staining reaction of activated B- and T-cells, in particular mantle zone B-cells, in the tonsil and intraepithelial T-cells in the appendix
• An at least weak to moderate staining reaction of the majority of the hepatic sinusoidal ECs
• An at least moderate, predominantly membranous staining reaction of the vast majority of neoplastic cells in the angiosarcoma
• No staining reaction of epithelial cells.
Sections from different levels of the TMAs were stained for CD31 in the NordiQC reference laboratory (Lab) using mmAb clone JC70A to ensure an appropriate and constant expression of CD31 throughout the slides to be circulated. Participants submitted their CD31 IHC staining protocols on the NordiQC homepage and received a pair of slides cut from the test TMAs. The participants performed the IHC assays for CD31 according to their submitted protocol and returned one of the slides to NordiQC. An expert assessor panel consisting of experienced pathologists and technicians performed the assessment. The assessor panel performed an anonymized consensus assessment by evaluating each slide on a microscope linked to a projector. Each assay was marked as “optimal,” “good,” “borderline,” or “poor” based on the technical quality and concordance to the staining pattern as outlined above. Optimal and good are considered sufficient, while borderline and poor are insufficient for diagnostic use.
Results
In the two runs (38/2013 and 46/2016), a total of 476 slides stained for CD31 were assessed (Table 1). The most commonly used antibody (Ab) was mouse monoclonal (mm) Ab clone JC70A applied in 257 cases, of which 73.9% provided a sufficient result (optimal or good). Optimal staining results could be obtained on all main platforms (Dako, Leica and Ventana). Among the Lab developed tests (LDTs), the highest proportion of optimal results was obtained by efficient heat induced epitope retrieval (HIER) in an alkaline buffer, TRS low pH (Dako) or DIVA pH 6 (Biocare), in combination with an appropriate calibration of the primary Ab and use of a sensitive 3-step detection system.
Table 1
| Clone and format | No. of slides | Optimal (%) | Good (%) | Insufficient (%) |
| JC70A Conc | 257 | 43.6 | 30.3 | 26.1 |
| JC70A RTU Dako | 100 | 57.0 | 35.0 | 8.0 |
| JC70A RTU Ventana | 82 | 30.5* | 20.7 | 48.8 |
| 1A10 RTU Leica | 10 | 0.0 | 0.0 | 100.0 |
| 1A10 Conc | 9 | 0.0 | 11.1 | 88.9 |
| Other | 18 | 16.7 | 16.7 | 66.7 |
| Total | 476 | 41.4 | 28.2 | 30.5 |
The mmAb clone JC70A in a ready to use (RTU) product from six vendors was used in 189 cases, of which 73.0% provided a sufficient result. The RTU products from Dako used in 100 cases gave sufficient results in 92.0%, of which 57% were optimal. An optimal result could both be obtained by the Dako recommended protocol settings and by Lab modified settings. The RTU product from Ventana used in 82 cases gave sufficient results in 51.2% and only 30.5% being optimal. An optimal result with the Ventana product was typically based on a Lab modified protocol using a 3-step detection system as OptiView or UltraView + amplification. In contrast, adherence to the vendor recommended protocol settings, which includes the 2-step visualization system Ultraview, could not provide any optimal staining reaction.
Only few participants used other antibodies than mmAb clone JC70A. The mmAb clone 1A10 was applied in 20 cases either as a concentrate (Conc) or an RTU product. However, none were optimal, only one was good, and 19 (95%) were insufficient. Nine slides were stained for CD31 using one of the clones EP3095, UC-CD31, BS50 or EP78 as a Conc or BC2 as an RTU product. None of these provided an optimal result and only BC2 gave good results (both of two stains).
The prevalent feature of insufficient staining results was a “too weak” or “false negative” staining reaction in cells and structures expected to be demonstrated (Figure 1). These patterns were observed in 98% of the insufficient results. Virtually all Labs were able to demonstrate CD31 in high level antigen expressing structures such as ECs of the large vessels in the appendix and portal tracts of the liver, whereas demonstration of CD31 in low level expressing structures as hepatic sinusoidal ECs and activated B-cells in the mantle zones of the tonsil were more challenging.
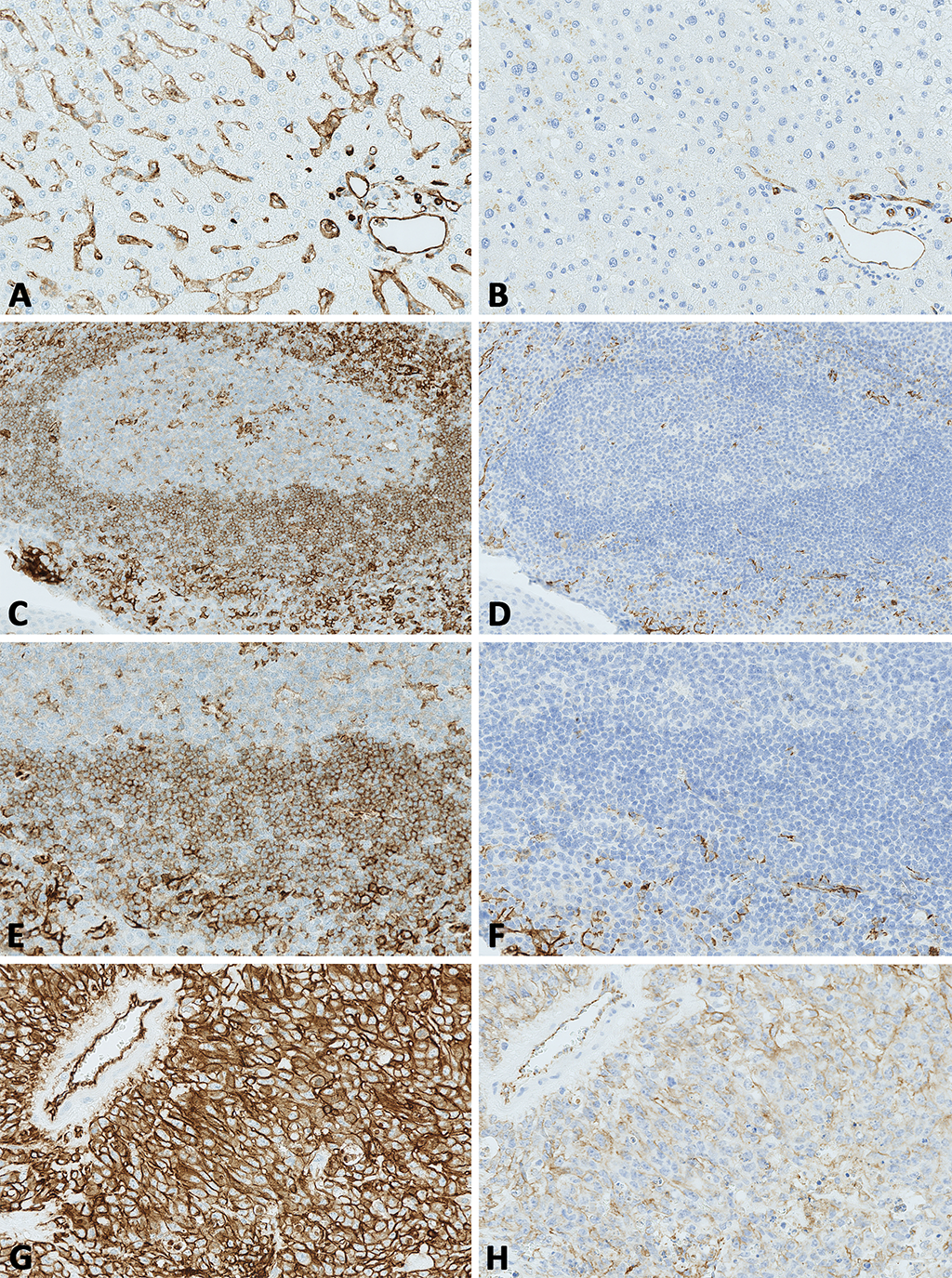
Figure 1: Optimal and insufficient staining results for CD31 as assessed by NordiQC.
Figure 1: Optimal and insufficient staining results for CD31 as assessed by NordiQC.
A. Optimal CD31 staining of the liver using the mmAb clone JC70A as a concentrate, HIER in an alkaline buffer and a 3-step multimer based detection system. Virtually all the hepatic sinusoidal endothelial cells show a weak to moderate, predominantly membranous staining reaction.
B. Same field as in A. Insufficient staining for CD31 of the liver using the mmAb clone JC70A in a protocol with a too low sensitivity. Endothelial cells lining large vessels are demonstrated, while the sinusoidal endothelial cells are false negative.
C. Optimal CD31 staining of the tonsil using the mmAb clone JC70A in an RTU format (GA610, Dako), with HIER in TRS High pH 9 for 30 min., a 3-step polymer-based detection kit, performed on the Dako Omnis stainer (vendor recommended protocol settings). Even at low magnification a distinct positive staining reaction of mantle zone B-cells, plasma cells and endothelial cells can be identified.
D. Same field as in C. Insufficient CD31 staining of the tonsil using the mmAb clone JC70A in an RTU format (IR610, Dako) but with laboratory modified protocol settings reducing HIER time and changing detection kit. The endothelial cells are weakly stained, the lymphocytes are false negative.
E. Higher magnification of the tonsil in C. A moderate and distinct membranous staining reaction is seen in the majority of mantle zone B-cells, while plasma cells and endothelial cells show an intense staining reaction.
F. Same field as in E, same protocol as in D. Insufficient CD31 staining of the tonsil. Only endothelial cells and plasma cells are demonstrated, while mantle zone B-cells are negative.
G. Optimal CD31 staining of the angiosarcoma using the same protocol as in C and E. Virtually all neoplastic cells show a distinct, predominantly membranous staining reaction.
H. Same field as in G, same antibody and protocol as in D and F. Insufficient CD31 staining of the angiosarcoma. The intensity and proportion of neoplastic cells demonstrated is significantly reduced compared to G. CD31 positive normal endothelial cells are demonstrated (top left) emphasizing that these cells cannot reliably be used as internal positive tissue control.
Foot note: All photos in the multi-panel are obtained by Hamamatsu Nano Zoomer 2.0-RS slide scanner at 20X (A-B, E-H) or 4X (C-D) magnification.
Discussion and Conclusion
In the NordiQC EQA, about 30% of all stains in the general module have been assessed as insufficient for diagnostic use [9]. About 90% of insufficient results are characterized by a “too weak” or “false negative” reaction, particularly in cells with a low epitope expression. In most cases, the major causes can be identified as less successful (poor and less robust) antibodies, poorly calibrated RTU products, stainer platform-dependent antibodies, insufficiently calibrated antibody dilutions, insufficient/erroneous epitope retrieval, less sensitive visualization systems, and others (e.g., retrieval induced impaired morphology, drying out phenomena, stainer platform-dependent protocol issues, platforms causing uneven staining reactions).
In the current EQA of CD31 assays, the less successful mmAb clone 1A10 and low sensitivity protocols were prevailing causes of insufficient staining results. Also a significant cause may be the lack of awareness of the immunohistochemical critical assay performance controls (iCAPCs) as it is essential to demonstrate an appropriate and successful level of the analytical sensitivity in the assay for CD31. Liver, tonsil and appendix combined are appropriate as positive and negative control tissues for CD3. In tonsil virtually all plasma cells and ECs lining large vessels should show a moderate to strong staining reaction, whereas the vast majority of mantle zone B-cells must show an at least weak to moderate staining reaction. The positive staining reaction of mantle zone B-cells with low-level CD31 expression serves as the iCAPC for the low limit of detection of CD31. In liver, the hepatic sinusoidal ECs are also suitable as iCAPC for CD31, due to low-level CD31 expression. No staining reaction should be seen in e.g., hepatocytes, appendiceal columnar epithelial cells and squamous epithelial cells in tonsil, all serving as negative tissue controls.
Also in this context, it has to be emphasized that these tissue controls should be used together as external on-slide control to confirm the IHC test reproducibility. Batch controls and internal controls (ECs in general) for CD31 cannot stand alone as in most instances they do not accurately monitor and assure the IHC analytical test performance [10].
In conclusion, the key points for CD31 immunoassays are as follows:
• Select a sensitive Ab clone, e.g., mmAb JC70A as Conc in an LDT or in a Dako/Agilent RTU format
• Do not use an insensitive clone like mmAb clone 1A10
• Use an optimized protocol based on HIER and a three-step polymer/multimer conjugate
• Use appropriate on-slide iCAPCs for CD31 (Liver, tonsil and appendix).
Original submitted files for images
Below are the links to the authors’ original submitted files for images.
Original image file for Figure 1
References
- The Role of Sialylated Glycans in Human Platelet Endothelial Cell Adhesion Molecule 1 (PECAM-1)-mediated Trans Homophilic Interactions and Endothelial Cell Barrier Function. J Biol Chem. 2016 Dec 9;291(50):26216-26225. doi:10.1074/jbc.M116.756502
- An immunologist's guide to CD31 function in T-cells. J Cell Sci. 2013 Jun 1;125(Pt 11):2343-52. doi:10.1242/jcs.124099
- PECAM-1-dependent neutrophil transmigration is independent of monolayer PECAM-1 signaling or localization. Blood. 2003 Apr 1;101(7):2816-25. doi:10.1182/blood-2002-08-2396
- Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J Immunol. 2002 Jul 15;169(2):983-92. doi:10.4049/jimmunol.169.2.983
- Capillarization of hepatic sinusoid by liver endothelial cell-reactive autoantibodies in patients with cirrhosis and chronic hepatitis. Am H Pathol. 2003 Oct;163(4):1275-89. doi:10.1016/S0002-9440(10)63487-6
- Effectiveness of Vascular Markers (Immunohistochemical Stains) in Soft Tissue Sarcomas. J Coll Physicians Surg Pak. 2018 May;28(5):352-356. doi:10.29271/jcpsp.2018.05.352
- CD31 immunoreactivity in carcinomas and mesotheliomas. Am J Clin Pathol. 1998 Sep;110(3):374-7. doi:10.1093/ajcp/110.3.374
- Utility of immunohistochemistry for endothelial markers in distinguishing epithelioid hemangioendothelioma from carcinoma metastatic to bone. Arch Pathol Lab Med. 2009 Jun;133(6):967-72. doi:10.1043/1543-2165-133.6.967
- Proficiency testing in immunohistochemistry--experiences from Nordic Immunohistochemical Quality Control (NordiQC). Virchows Arch. 2016 Jan;468(1):19-29. doi:10.1007/s00428-015-1829-1
- Standardization of positive controls in diagnostic immunohistochemistry: recommendations from the International Ad Hoc Expert Committee. Appl Immunohistochem Mol Morphol. 2015 Jan;23(1):1-18. doi:10.1097/PAI.0000000000000163

